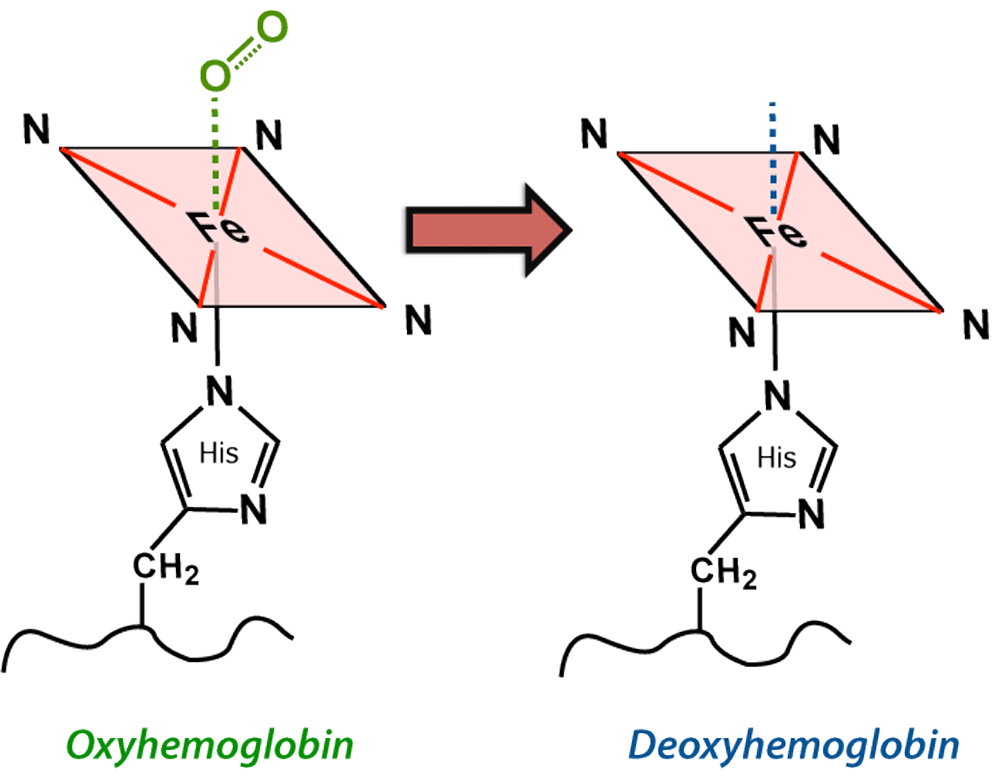
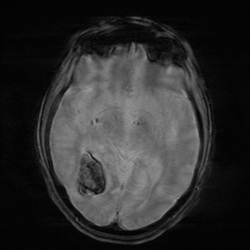
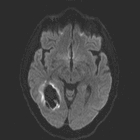
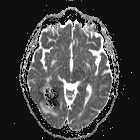
Local field distortions due to deoxy-Hb within
Local field distortions due to deoxy-Hb within RBCs dephases nearby H2O molecules, causing
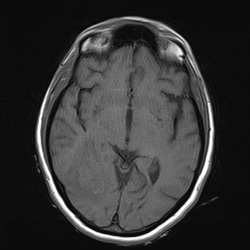
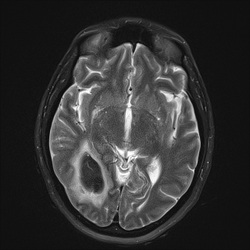
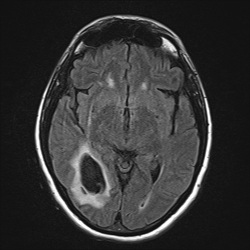
loss of signal on T2/T2*-weighted images.
This marked dephasing due to concentrated deoxy-Hb spills over into diffusion-weighted images, producing the so-called "T2-blackout effect." In reality, diffusion remains restricted in the center of acute hematomas, just as it does in hyperacute ones. However, the expected high signal on DW images representing restricted diffusion is masked by susceptibility-induced signal loss.
Although T2 and T2* are significantly shortened by the acute hematoma, T1 values are relatively unaffected. This is because the conformation of the globin proteins do not allow water molecules close access to the paramagnetic centers of deoxy-Hb. Outer sphere relaxation mechanisms predominate, resulting in strong effects on T2 with little or no change in T1 values. Thus, as with hyperacute hemorrhages, T1-weighted images remain isointense to brain.
Important Note: The discussion above primarily applies to higher field (≥ 0.5 T) systems where imaging findings are dominated by the paramagnetic effects of hemoglobin. At low and intermediate fields some important differences in imaging appearances are noted. See this Q&A for a more detailed analysis.
Advanced Discussion (show/hide)»
Ambient tissue oxygen levels affect the evolution of hemorrhage. Either too high or too low tissue oxygen will result in delayed evolution between oxy-Hb and deoxy-Hb.
Some hematomas in contact with healthy capillary networks remain exposed to relatively high levels of oxygen (at least peripherally). Examples include hematomas adjacent to vascular malformations, intramuscular hematomas, and subarachnoid hemorrhage. In these cases high ambient O2 keeps more blood in the oxyhemoglobin state with slower transition to deoxyhemoglobin. Methemoglobin levels are also slow to rise, as oxygen powers the methemoglobin reductase systems driving equilibrium back to deoxyhemoglobin.
Paradoxically, too low levels of ambient oxygen may also result in delayed hematoma evolution. Examples include intratumoral hemorrhage and hemorrhagic infarctions. Here blood may stay in the deoxyhemoglobin state for a longer period of time, because ambient O2 or peroxide is needed as an electron receptor to oxidize heme iron from its ferrous (Fe+2) state in deoxyhemoglobin to the ferric (Fe+3) state in methemoglobin.
Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology 1993; 189:15-26.
Gomori JM, Grossman RI. Mechanisms responsible for the MR appearance and evolution of intracranial hemorrhage. Radiographics 1988; 8:427-440.
Hiwatashi A, Kinoshita T, Moritani T et-al. Hypointensity on diffusion-weighted MRI of the brain related to T2 shortening and susceptibility effects. AJR Am J Roentgenol 2003; 181:1705-9.
Maldjian JA, Listerud J, Moonis G, Siddiqi F. Computing diffusion rates in T2-dark hematomas and areas of low T2 signal. AJNR Am J Neuroradiol 2001;22:112–128.
Silvera S, Oppenheim C, Touzé E, et al. Spontaneous intracerebral hematoma on diffusion-weighted images: influence of T2-shine-through and T2-blackout effects. AJNR Am J Neuroradiol 2005; 26:236-241.
What are the different forms of hemoglobin and why do they have different magnetic properties?
I know what T2-shine-through is, but what is T2-blackout?
How does the MR appearance of blood differ at low and very low magnetic fields?