|
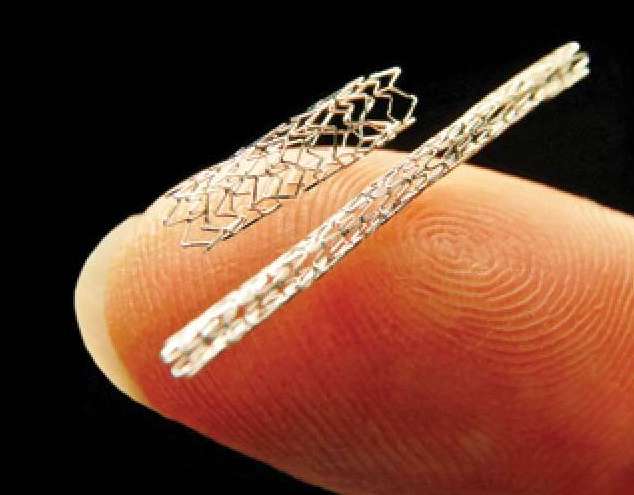
Coronary Artery Stents
Coronary artery stents are primarily used to prevent restenosis after balloon angioplasty. Before 2000 most stents were made of nonferromagnetic stainless steel, but now many are made of other non-ferrous metals including Nitinol®, cobalt-chromium, or platinum-chromium. Multiple studies have demonstrated that these stents are MR-conditional at up to 3.0T and that notwithstanding warnings by some manufacturers, MR scanning may take place immediately after implantation.
|
Cardiac Closure and Occluder Devices
A number of catheter-deployed devices are available, all of which are made of non-ferromagnetic materials and are MR Conditional. The include those designed for:
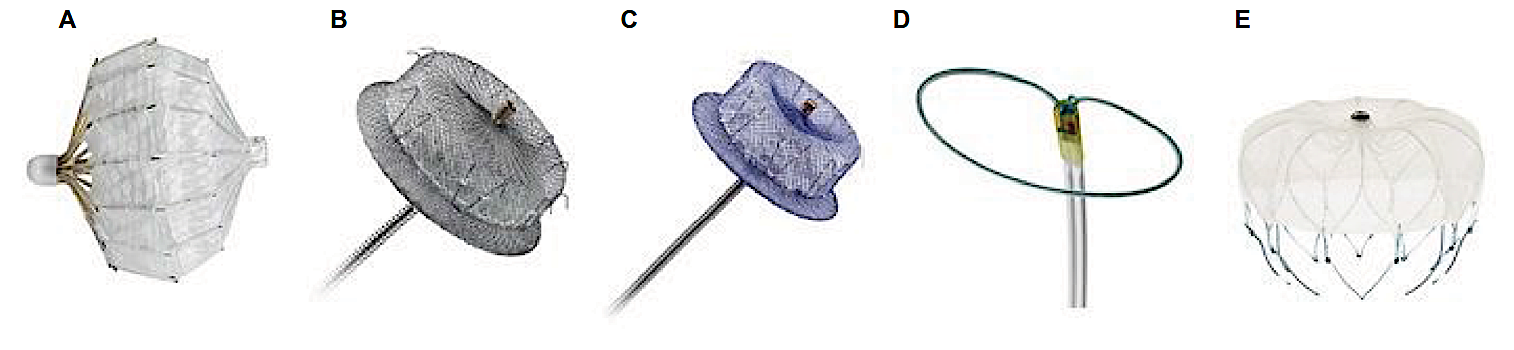
- Closure of atrial septal defect (ASD), ventricular septal defect (VSD), and patent foramen ovale (PFO) — Abbott Amplatzer® Septal Occluder, Figulla® Flex II ASD Occluder, Hyperion® PFO Occluder
- Closure of patent ductus arteriosus (PDA) -- Cook® PDA Occluder Coil, PFM Nit-Occlud® PDA Occlusion System
- Obliteration of left atrial appendage (LAA) -- Watchman® device, Amplatzer® Amulet AAA Occluder, AtriClip® (looks like a hairpin), LARIAT® device
 "MR Conditional" V-wave shunt device placed in the inter-atrial septum
"MR Conditional" V-wave shunt device placed in the inter-atrial septum
Inter-Atrial Shunts
Some patients with severe left-sided heart failure may benefit from reducing left atrial pressure by decompression into the right atrium. This can be accomplished by placing a passive shunt via fluoroscopy in the interatrial septum. Two such devices are commercially available: the Ventura™ Interatrial Shunt System (VWave) and the Interatrial Shunt Device (IASD)® System (Corvia). Both are made of non-ferromagnetic materials and should be rated as MR Conditional. There are no moving parts or flaps. The relatively narrow channel has high resistance allowing blood flow from the left to the right only.
Some patients with severe left-sided heart failure may benefit from reducing left atrial pressure by decompression into the right atrium. This can be accomplished by placing a passive shunt via fluoroscopy in the interatrial septum. Two such devices are commercially available: the Ventura™ Interatrial Shunt System (VWave) and the Interatrial Shunt Device (IASD)® System (Corvia). Both are made of non-ferromagnetic materials and should be rated as MR Conditional. There are no moving parts or flaps. The relatively narrow channel has high resistance allowing blood flow from the left to the right only.
Mechanical Circulatory Assist Devices
|
As expected, essentially all mechanical circulatory assist devices for heart failure are considered "MR Unsafe". These include left and right ventricular assist devices (LVAD/RVAD's), Extracorporeal Membrane Oxygenation (ECMO) systems, Intra-aortic balloon pumps (IABP), and other percutaneous systems (e.g., Impella, HeartMate, TandemHeart). One manufacturer (Teleflex) allows Conditional MR imaging with its IABP in place, provided the device is stopped and its external components removed, leaving the catheter and balloon in place. I can't believe anyone would ever do this but apparently you can.
|
Pulmonary Artery Sensor
|
Currently only one FDA/CE-approved pulmonary artery remote pressure sensor exists, the CardioMEMS® by Abbott. The CardioMEMS® is placed via right heart catheterization in a lower lobe pulmonary artery, held in place by thin wire loops. The device is passive, not requiring an internal power source, being stimulated by a superficial radiofrequency generator that also receives and records pressure measurements. It is MR Conditional.
|
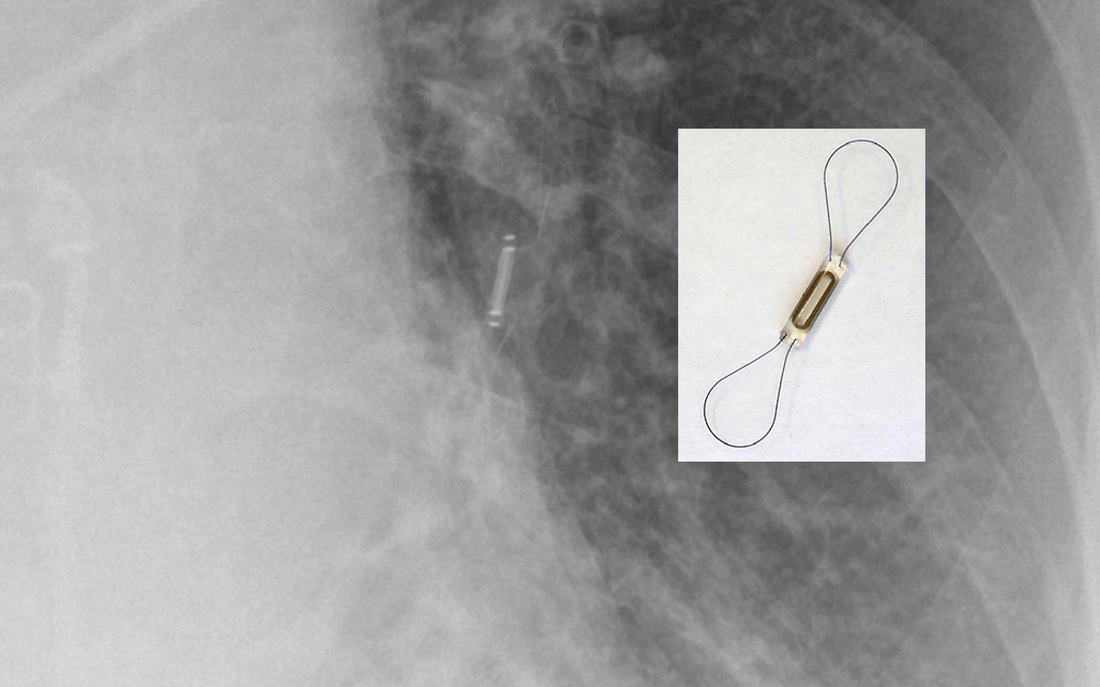
"MR Conditional" CardioMEMS® pulmonary artery pressure sensor. (Modified from DB Chonde, Radiopaedia.org)
|
Left Ventricular Reconstruction Device
 "MR Conditional" Revivent® System used to close up an akinetic anterior wall scar
"MR Conditional" Revivent® System used to close up an akinetic anterior wall scar(From Nijenhuis under CC-BY)
In heart failure after an infarction, the heart commonly changes shape due to akinetic segments and aneurysm formation. The Revivient® TC Ventricular Enhancement System (BioVentrix) consists of cloth-covered MR Conditional titanium anchor pairs joined by an adjustable length polyethylene tether. The anchors are is implanted using a catheter and mini-thoracotomy. The system improves cardiac function by excluding scarred myocardium and reducing ventricular volume, radius and wall tension.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring inchronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–66.
Hendriks T, Schurer RAJ, Ali LA, et al. Left ventricular restoration devices post myocardial infarction. Heart Failure Reviews 2018; 23:871-883.
Honjol Y, Rajkumar VS, Parent-Harvey C, et al. Current view and prospect: implantable pressure sensors for health and surgical care. Med Devices Sens 2020; 3:e10068. [DOI LINK]
Hug J, Nagel E, Bornstedt A, et al. Coronary arterial stents: Safety and artifacts during MR imaging. Radiology 2000;216:781–787.
Nijenhuis VJ, Sanchis L, van der Heyden JAS, et al. The last frontier: transcatheter devices for percutaneous or minimally invasive treatment of chronic heart failure. Neth Heart J 2017; 25:536-544.
Schmidt T, Abbott JD. Coronary stents: history, design, and construction. J Clin Med 2018; 7:126 [DOI LINK]
Swaans MJ, Wintgens LIS, Alipour A, et al. Percutaneous left atrial appendage closure devices: safety, efficacy, and clinical utility. Medical Devices: Evidence and Research 2016; 9:309-316.
Syed MA, Carlson K, Murphy M, et al. Long-term safety of cardiac magnetic resonance imaging performed in the first few days after bare-metal stent implantation. J Magn Reson Imaging 2006;24:1056–1061.
Teleflex®. MRI labeling information for Arrow® IAB and IABP Systems. Teleflex Inc, 2019. (The document tells how MRI may be conditionally performed by stopping and disconnecting the mechanical pump with concomitant risks of hypotension and thrombus formation.)
Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring inchronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–66.
Hendriks T, Schurer RAJ, Ali LA, et al. Left ventricular restoration devices post myocardial infarction. Heart Failure Reviews 2018; 23:871-883.
Honjol Y, Rajkumar VS, Parent-Harvey C, et al. Current view and prospect: implantable pressure sensors for health and surgical care. Med Devices Sens 2020; 3:e10068. [DOI LINK]
Hug J, Nagel E, Bornstedt A, et al. Coronary arterial stents: Safety and artifacts during MR imaging. Radiology 2000;216:781–787.
Nijenhuis VJ, Sanchis L, van der Heyden JAS, et al. The last frontier: transcatheter devices for percutaneous or minimally invasive treatment of chronic heart failure. Neth Heart J 2017; 25:536-544.
Schmidt T, Abbott JD. Coronary stents: history, design, and construction. J Clin Med 2018; 7:126 [DOI LINK]
Swaans MJ, Wintgens LIS, Alipour A, et al. Percutaneous left atrial appendage closure devices: safety, efficacy, and clinical utility. Medical Devices: Evidence and Research 2016; 9:309-316.
Syed MA, Carlson K, Murphy M, et al. Long-term safety of cardiac magnetic resonance imaging performed in the first few days after bare-metal stent implantation. J Magn Reson Imaging 2006;24:1056–1061.
Teleflex®. MRI labeling information for Arrow® IAB and IABP Systems. Teleflex Inc, 2019. (The document tells how MRI may be conditionally performed by stopping and disconnecting the mechanical pump with concomitant risks of hypotension and thrombus formation.)
Related Questions
Are all stents and stent grafts safe to scan? Isn't there a waiting period after implantation?
Are all stents and stent grafts safe to scan? Isn't there a waiting period after implantation?