As mentioned in the prior Q&A, the iron centers of deoxyhemoglobin (deoxy-Hb) are each maintained in the ferrous (Fe+2) state, while those of methemoglobin (met-Hb) exist in the ferric (Fe+3) state. The transition from deoxy-Hb to met-Hb thus requires the loss of an electron from each iron center.
|
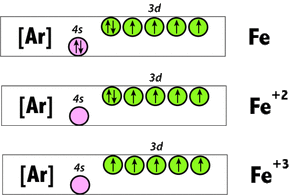
This paradox can be easily resolved by looking at the electronic structures of neutral (Fe), ferrous (Fe+2), and ferric (Fe+3) iron. Ferrous (Fe+2) iron has 6 electrons in its 3d shell, 2 of which are paired. This results in 4 unpaired electrons in this valence state. When one of these electrons is lost to form ferric (Fe+3) iron, 5 unpaired electrons then exist in the 3d shell, making met-Hb slightly more paramagnetic than deoxy-Hb. |
Advanced Discussion (show/hide)»
The principle explaining the distribution of electrons in the various forms of iron is described in 1st year chemistry courses and is known as Hund's Rule of Maximum Multiplicity. This observational rule states that each orbital in a subshell should be singly occupied by one electron before any orbital is doubly occupied, and that all electrons in singly occupied orbitals should have the same spin.
Although this principle works for nearly all lower state atomic systems, including those of Fe, interesting counterexamples exist where the rule is violated. These include the 3snd series of magnesium and certain non-Kekule hydrocarbons where spin-polarization effects predominate.
Why is methemoglobin bright on T1-weighted images?