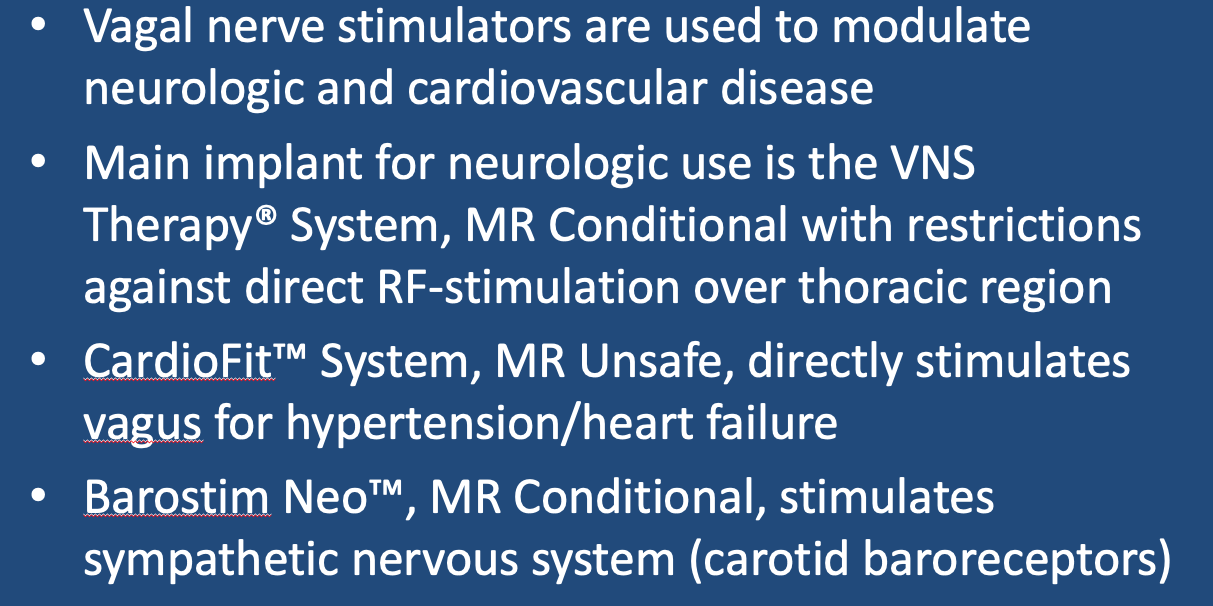
LivaNova VNS Therapy® System
LivaNova VNS Therapy® System
VNS systems resemble cardiac pacemakers. They consist of a battery and pulse generator (typically implanted subcutaneously in the upper chest) and a lead with terminal electrodes placed adjacent to a segment of the vagus nerve (usually in the mid-neck).
The standard stimulation regimen is 30 seconds every 5 minutes, but can be adjusted by an external programming device on a patient-by-patient basis. Newer models also detect changes in heart rate (that can increase immediately before a seizure) and automatically apply extra stimulating pulses. An external hand-held magnet placed over the pulse generator can temporarily increase stimulation rate when needed during a seizure
 CardioFit™ Vagal Nerve Stimulator
CardioFit™ Vagal Nerve Stimulator
 Barostim Neo™ System (from Wilks et al under CC-BY)
Barostim Neo™ System (from Wilks et al under CC-BY)
Advanced Discussion (show/hide)»
I always found it curious that some implanted stimulators defined restricted areas for body coil RF-irradiation that don't seem to make sense. For example, LivaNova cites an RF-exclusion zone of C7-L3 for their VNS. Why were these vertebral levels chosen and why is the exclusion zone so asymmetric compared to the typical location of the implanted pulse generator at T2-T4 and upwardly coursing electrodes?
The paper by Vaughn et al (2004) shows that for modern bird-cage RF body coils, a 3 dB drop of in RF magnitude (corresponding to about a 50% decrease in RF power) occurs about 10-15 cm from isocenter. So placing isocenter at C6 (permitted under LivaNova's guidelines) appreciable RF power to the IPG and lead(s) would still occur. Or alternatively, placing isocenter at L2 would seem to have little or no effect on the VNS components compared to isocenter placement over the (permitted areas of the) neck or head.
My conjecture is LivaNova chose these specific levels not as a result of rigorous scientific analysis, but was simply mirroring the so-called "thoracic exclusion zone" that had been applied to many non-conditional cardiac pacemakers in the 2000-2015 era. For this reason I would personally not be too reluctant to scan at least the lower thoracic region of a patient with a VNS device.
The Physicians Manual below contains additional manufacturer's recommendations for how to handle several special cases, including systems with broken leads and orphaned devices or leads.
Anand IS, Konstam MA, Klein HU, et al. Comparison of symptomatic and functional responses to vagus nerve stimulation in ANTHEM-HF, INOVATE-HF and NECTAR-HF. ESC Heart Failure 2020; 7:76-84. [DOI LINK]
CVRx, Inc. MRI Use Instructions (for BarostimNeo). August 2019. (Before scanning always check manufacturer's web site for most recent version of this document and current recommendations)
LivaNova. MRI with the VNS Therapy® System. October 2019. (Before scanning always check manufacturer's web site for most recent version of this document and current recommendations)
Vaughan JT, Adrian G, Snyder CJ, et al. Efficient high-frequency body coil for high-field MRI. Magn Reson Med 2004; 52:851-859. [DOI LINK]
Wilks SJ, Hara SA, Ross EK, et al. Non-clinical and pre-clinical testing to demonstrate safety of the Barostim Neo electrode for activation of carotid baroreceptors in chronic human implants. Front Neurosci 2017; 11:438. [DOI LINK]
What precautions must be taken when scanning patients with an InterStim device?
Can patients with deep brain stimulators be scanned?