In the late 2000s several medical device companies began to develop cardiac pacemakers that were MR Conditional. The first to market was Medtronic's EnRhythm™ MRI SureScan Pacemaker System (which received CE Mark approval in November 2008). The device was modified and rebranded as RevoMRI™ SureScan System, receiving FDA approval in February. 2011.
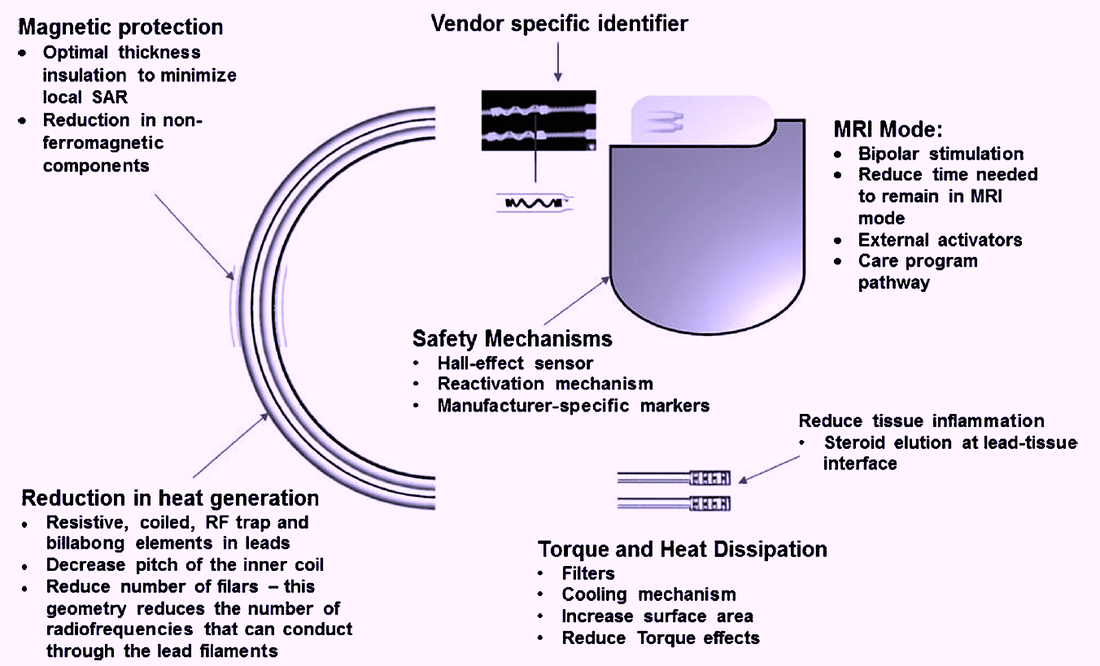
Multiple components of the classic pacemaker system had be re-engineered to allow the devices to be MR Conditional. The most important changes included the use of Hall-effect sensors instead of reed switches to toggle pacing mode, software reprogramming for operation in "MRI mode", and innovative lead design to minimize heat generation and standing wave currents. These and other modifications are illustrated below.
Multiple components of the classic pacemaker system had be re-engineered to allow the devices to be MR Conditional. The most important changes included the use of Hall-effect sensors instead of reed switches to toggle pacing mode, software reprogramming for operation in "MRI mode", and innovative lead design to minimize heat generation and standing wave currents. These and other modifications are illustrated below.
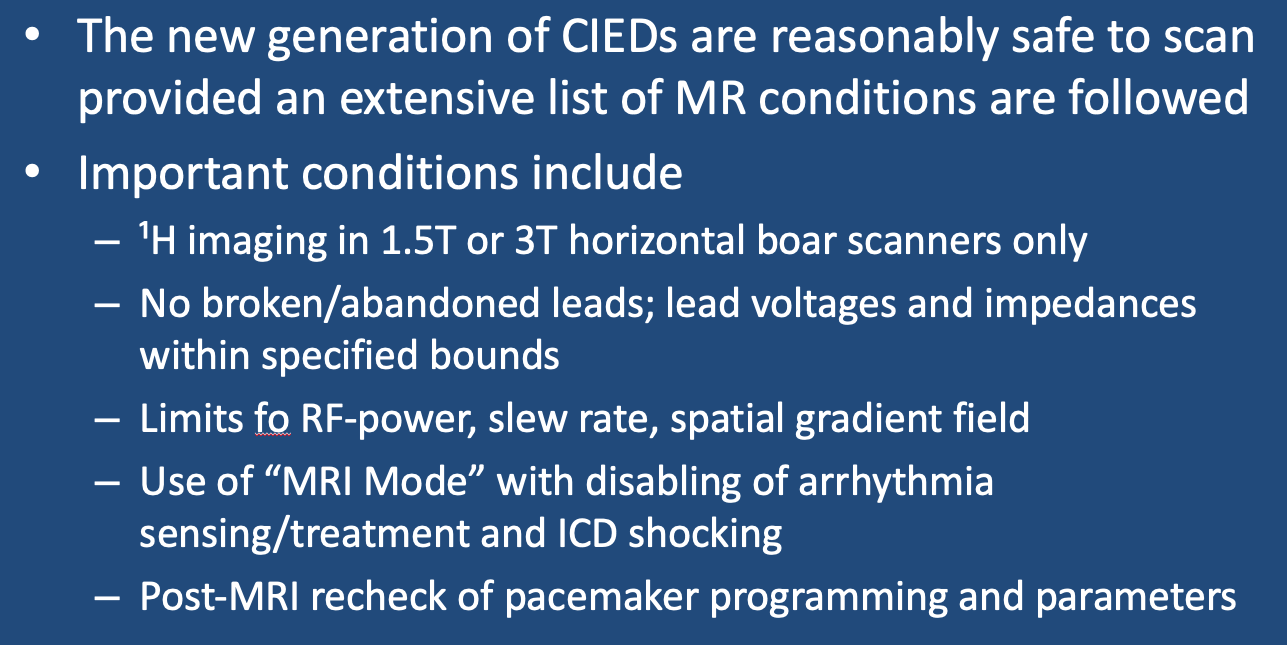
Today, at least a half-dozen companies manufacture MR Conditional pacemakers and ICD's which are becoming widely implanted. Being MR Conditional means they are reasonably safe to scan provided specific conditions are followed. Such conditions will vary by the particular device and manufacturer. These conditions are generally quite extensive and must be followed precisely. As always, check with the manufacturer's web site for the most current requirements. Below are some generic conditions that typically accompany MR Conditional Cardiovascular Implanted Electronic Devices (CIEDs):
- Need/risk assessment. Does the patient really need an MRI? A radiologist may need to be consulted to determine whether an alternative (non-MRI) exam may provide equal or superior diagnostic results. If a need is demonstrated, a cardiologist should determine whether or not the patient is pacemaker-dependent (as some different conditions and risks may apply).
- Device identification. The exact model of Pacemaker/ICD, leads used, and date of placement must be established with certainty. Most manufacturers recommend waiting 6 weeks to allow for maturation of the lead-myocardial interface prior to MRI. Leads must also be MR Conditional and appropriately paired with the proper the pulse generator component. (Note that an MR conditional lead from one company coupled with an MR conditional pulse generator from a different company renders the entire device Non-conditional).
- Obtain or review a recent chest x-ray. This will confirm several important requirements: a) that the pulse generator is implanted in the left or right pectoral region (anywhere else is contraindicated); b) that there are no lead extenders or adaptors; c) that no abandoned or broken leads are present; and d) there are no additional nearby electronic implants such as neural stimulators.
- Evaluation and interrogation of the CIED. A cardiologist or skilled electrophysiology technologist should remotely interrogate the device to be insure the battery is not near the end of its life and that lead impedances and capture threshold values lie within specified ranges. (For ICDs, defibrillation lead impedances should also be checked). It should also be documented that no diaphragmatic pacing is noted at the highest lead voltages.
- MR Equipment and Protocols. All current MR-conditional pacemakers are restricted to ¹H imaging in horizontal bore cylindrical magnets operating at 1.5 or 3.0T. Usually only the supine or prone patient positions are allowed. Limits for maximum spatial gradient, gradient slew rate, and RF power dissipation measured by average SAR or maximum B1+RMS must be followed. Limitations may be more stringent if the thorax is imaged, including restrictions on the use of local transmit/receive coils and parallel RF transmission.
- CIED set to operate in appropriate pacing mode for MRI. The proper pacing mode should be determined by the cardiac electrophysiology service. Most MR-conditional CIED's have an "MRI Mode" with a stepwise algorithm allowing the best parameters to be selected. (Note that this is not the same as "magnet mode" which sets resets the CIED to a device-specific pacing mode that may or may not be the most appropriate for a given patient in MRI). Magnet mode and arrhythmia detection/therapies should be disabled. For non-pacemaker dependent patients, usually either a non-pacing (ODO/OVO/OAO) or inhibited mode (DDI/VVI/AAI) is appropriate, but can be an asynchronous mode (DOO/VOO/AOO) for some patients Pacemaker-dependent patients will generally be placed an asynchronous mode set at a level so as not to compete with their intrinsic heart rates. For patients with ICD's, tachy therapy shocking should be disabled as well as capacitor maintenance.
- Before, During, and After MRI. All paperwork and consents must be carefully reviewed before the patient enters the scanner room. Continuous visual monitoring with EKG, pulse oximetry and blood pressure should be performed. An external defibrillator should available on standby. After the exam, MRI-specific settings should be returned to pre-MRI values. Someone from the cardiac service should recheck all pacer parameters, including rechecking lead voltages, impedances, and sensing thresholds.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Ferreira AM, Costa F, Tralhão A, et al. MRI-conditional pacemakers: current perspectives. Med Devices: Evidence and Research 2014; 7:115-124.
Mulpuru SK, Madhavan M, McLeod CJ, et al. Cardiac pacemakers: function, troubleshooting, and management. J Am Coll Cardiol 2017; 69:189-210. [DOI LINK]
Muthalaly RG, Nerlekar N, Ge Y, et al. MRI in patients with cardiac implantable electronic devices. Radiology 2018; 289:281-292. [DOI LINK]
Poh PG, Lieu C, Yeo C, et al. Cardiovascular implantable electronic devices: a review of the dangers and difficulties in MR scanning and attempts to improve safety. Insights Imaging 2017; 8:405-418. [DOI LINK]
Shinbane JS, Colletti PM, Sherlock FG. Magnetic resonance imaging in patients with cardiac pacemakers: era of “MR Conditional” designs. J Cardiovasc Magn Reson 2011;13:63. [DIRECT LINK]
Vigen KK, Reeder SB, Hood MN, et al. Recommendations for imaging patients with cardiac implantable electronic devices (CIEDs). J Magn Reson Imaging 2020. [DOI LINK]
Ferreira AM, Costa F, Tralhão A, et al. MRI-conditional pacemakers: current perspectives. Med Devices: Evidence and Research 2014; 7:115-124.
Mulpuru SK, Madhavan M, McLeod CJ, et al. Cardiac pacemakers: function, troubleshooting, and management. J Am Coll Cardiol 2017; 69:189-210. [DOI LINK]
Muthalaly RG, Nerlekar N, Ge Y, et al. MRI in patients with cardiac implantable electronic devices. Radiology 2018; 289:281-292. [DOI LINK]
Poh PG, Lieu C, Yeo C, et al. Cardiovascular implantable electronic devices: a review of the dangers and difficulties in MR scanning and attempts to improve safety. Insights Imaging 2017; 8:405-418. [DOI LINK]
Shinbane JS, Colletti PM, Sherlock FG. Magnetic resonance imaging in patients with cardiac pacemakers: era of “MR Conditional” designs. J Cardiovasc Magn Reson 2011;13:63. [DIRECT LINK]
Vigen KK, Reeder SB, Hood MN, et al. Recommendations for imaging patients with cardiac implantable electronic devices (CIEDs). J Magn Reson Imaging 2020. [DOI LINK]
Related Questions
What precautions must be taken to scan patients with older/legacy/non-conditional pacemakers?
What precautions must be taken to scan patients with older/legacy/non-conditional pacemakers?