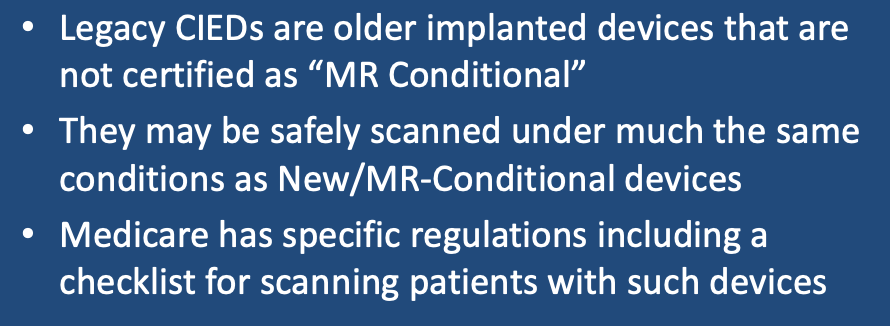
Over the last 20 years it has become increasingly recognized that certain older/legacy/non-conditional pacemakers and ICDs may indeed be safely scanned in MRI provided certain precautions are taken. Several professional societies, including the Heart Rhythm Society (HRS, 2017) and the International Society of Magnetic Resonance in Medicine (ISMRM, 2020), have addressed this issue through expert consensus statements and suggested protocols. Even the US Centers for Medicare & Medicaid Services (CMS, 2018) have weighed in and provided recommendations and requirements for coverage for these patients.
Essentially the same procedures described in the prior Q&A for MR Conditional CIEDs should be followed for non-conditional ones. A few points of emphasis should perhaps be added:
- Explaining the risks and benefits to the patient should not be abbreviated and be thoroughly documented in the medical records. Although research supports that such scans can be done safely, I personally believe non-conditional CIED patients are assuming a slightly greater risk. This is especially true for pacemaker-dependent patients.
- Equipment and protocols: I'd recommend staying at 1.5T, Normal Operating Mode, and keeping SAR levels as low as possible.
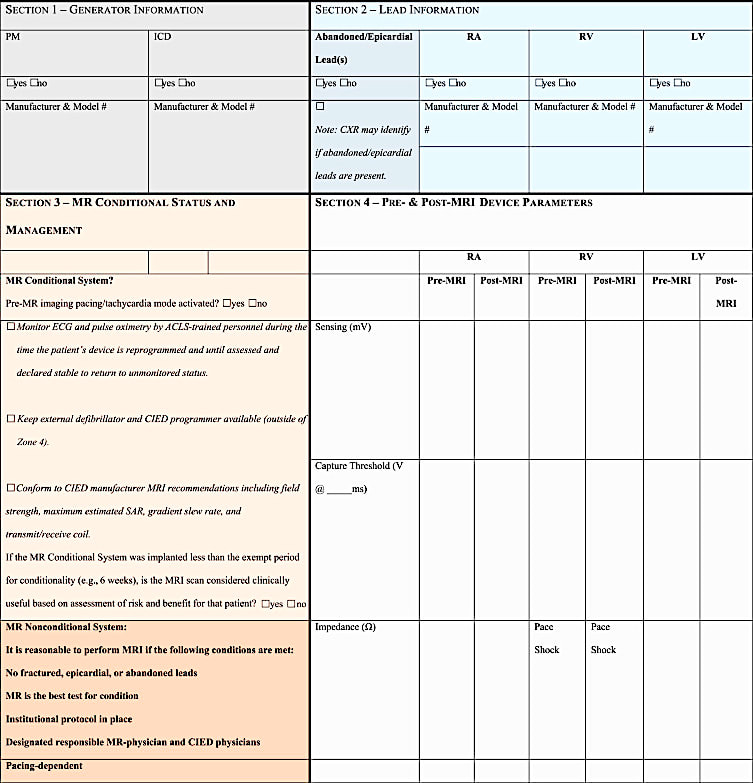
- Be particularly aware that if the non-conditional device contains a reed switch, it will likely close during when placed in the magnetic field, and then may open and close unpredictably. The device should be reprogrammed to exclude action of the reed switch prior to scanning.
- If you are in the USA and want to be paid for your work by Medicare/Medicaid, you must follow their rules explicitly. This includes documentation of every procedural step using a checklist, scanning only at 1.5T in Normal Operating Mode, and not scanning any patients with broken or abandoned leads. Direct supervision of the exam by a a qualified physician, nurse practitioner or physician assistant with expertise CIEDs is also required.
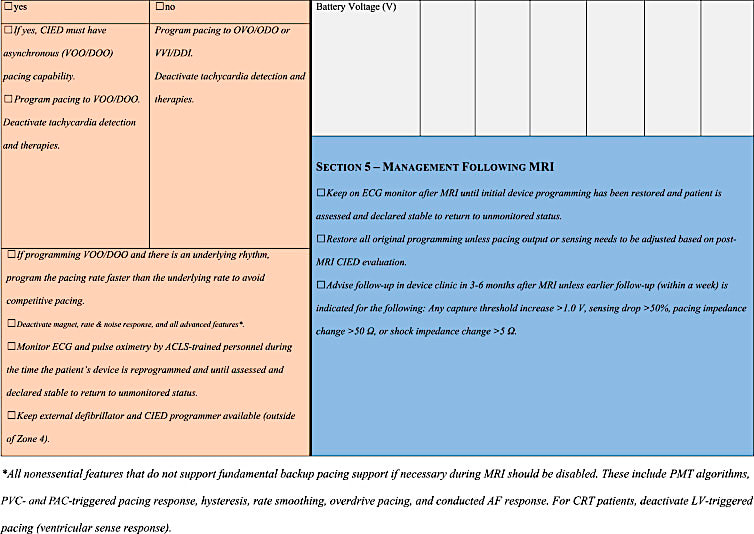
A Checklist Recommended by the Heart Rhythm Society (2017) appears below
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Centers for Medicare & Medicaid Services. Decision memo for magnetic resonance imaging (MRI) (CAG-00399R4). 2018. (A scholarly and lengthy review focused heavily on MRI of non-conditional CIEDs, including responses to comments made about policy decisions and recommendations) [DIRECT LINK]
Gupta SK, Ya’qoub L, Wimmer AP, et al. Safety and clinical impact of MRI in patients with non-MRI-conditional cardiac devices. Radiology: Cardiothoracic Imaging 2020; 2(5):e200086. [DOI LINK]
Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Health Rhythm 2017; 14:e97-e153. [DOI Link]
Jacob S, Panaich SS, Maheshwari R, et al. Clinical applications of magnets on cardiac rhythm management devices. Europace 2011; 13: 1222-1230.
Luechinger R, Duru F, Zeijlemaker VA, et al. Pacemaker reed switch behavior in 0.5, 1.5, and 3.0 Tesla magnetic resonance imaging units: are reed switches always closed in strong magnetic fields? Pacing Clin Electrophysiol 2002; 25:1419-23. (reed switches closed at 8G±2 and opened at 7G±2, supporting the long-held belief of the 5G line as still valid)
Maralani PJ, Schieda N, Hecht EM, et al. MRI safety and devices: an update and expert consensus. J Magn Reson Imaging 2019; 51:657-674. [DOI LINK]
Mulpuru SK, Madhavan M, McLeod CJ, et al. Cardiac pacemakers: function, troubleshooting, and management. J Am Coll Cardiol 2017; 69:189-210. [DOI LINK]
Muthalaly RG, Nerlekar N, Ge Y, et al. MRI in patients with cardiac implantable electronic devices. Radiology 2018; 289:281-292. [DOI LINK]
Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm 2009; 6:138–143. [DOI LINK]
Poh PG, Lieu C, Yeo C, et al. Cardiovascular implantable electronic devices: a review of the dangers and difficulties in MR scanning and attempts to improve safety. Insights Imaging 2017; 8:405-418. [DOI LINK]
Rajah P, Kay F, Bolen M, et al. Cardiac magnetic resonance in patients with cardiac implantable electronic devices. Challenges and solutions. J Thor Imaging 2020; 35:W1-W17. [DOI LINK]
Vigen KK, Reeder SB, Hood MN, et al. Recommendations for imaging patients with cardiac implantable electronic devices (CIEDs). J Magn Reson Imaging 2020. [DOI LINK]
Centers for Medicare & Medicaid Services. Decision memo for magnetic resonance imaging (MRI) (CAG-00399R4). 2018. (A scholarly and lengthy review focused heavily on MRI of non-conditional CIEDs, including responses to comments made about policy decisions and recommendations) [DIRECT LINK]
Gupta SK, Ya’qoub L, Wimmer AP, et al. Safety and clinical impact of MRI in patients with non-MRI-conditional cardiac devices. Radiology: Cardiothoracic Imaging 2020; 2(5):e200086. [DOI LINK]
Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Health Rhythm 2017; 14:e97-e153. [DOI Link]
Jacob S, Panaich SS, Maheshwari R, et al. Clinical applications of magnets on cardiac rhythm management devices. Europace 2011; 13: 1222-1230.
Luechinger R, Duru F, Zeijlemaker VA, et al. Pacemaker reed switch behavior in 0.5, 1.5, and 3.0 Tesla magnetic resonance imaging units: are reed switches always closed in strong magnetic fields? Pacing Clin Electrophysiol 2002; 25:1419-23. (reed switches closed at 8G±2 and opened at 7G±2, supporting the long-held belief of the 5G line as still valid)
Maralani PJ, Schieda N, Hecht EM, et al. MRI safety and devices: an update and expert consensus. J Magn Reson Imaging 2019; 51:657-674. [DOI LINK]
Mulpuru SK, Madhavan M, McLeod CJ, et al. Cardiac pacemakers: function, troubleshooting, and management. J Am Coll Cardiol 2017; 69:189-210. [DOI LINK]
Muthalaly RG, Nerlekar N, Ge Y, et al. MRI in patients with cardiac implantable electronic devices. Radiology 2018; 289:281-292. [DOI LINK]
Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm 2009; 6:138–143. [DOI LINK]
Poh PG, Lieu C, Yeo C, et al. Cardiovascular implantable electronic devices: a review of the dangers and difficulties in MR scanning and attempts to improve safety. Insights Imaging 2017; 8:405-418. [DOI LINK]
Rajah P, Kay F, Bolen M, et al. Cardiac magnetic resonance in patients with cardiac implantable electronic devices. Challenges and solutions. J Thor Imaging 2020; 35:W1-W17. [DOI LINK]
Vigen KK, Reeder SB, Hood MN, et al. Recommendations for imaging patients with cardiac implantable electronic devices (CIEDs). J Magn Reson Imaging 2020. [DOI LINK]
Related Questions
Aren't new pacemakers safe to scan in MRI? Any limitations?
Aren't new pacemakers safe to scan in MRI? Any limitations?