The form of paramagnetism exhibited by gadolinium compounds derives from electrons, not protons, and is known as Curie paramagnetism. Because electrons have the same spin (½) but a much smaller size than protons, their gyromagnetic ratios are 657 times larger. If these electrons remain unpaired in shells or bonding orbitals, the unbalanced spins produce a strong magnetic moment capable of inducing magnetic relaxation in nearby nuclei. This is the origin of the bulk paramagnetism possessed by elements such as gadolinium.
|
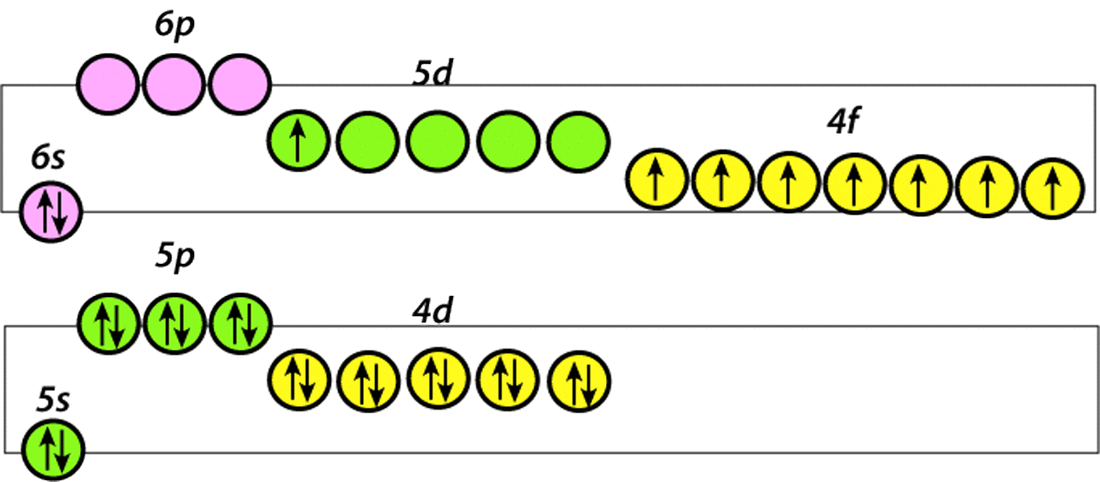
The electronic structure of the neutral Gd atom is shown right. Note the 7 unpaired electrons in its 4f subshell that account for the element's strong paramagnetism. In its ionized state, Gd+3 donates its 6s² and 5d¹ electrons for bonding, leaving its 4f7 electron shell intact. The powerful magnetic moment of Gd is therefore largely maintained even when chelated to a ligand such as DTPA in a contrast agent formulation. |
Advanced Discussion (show/hide)»
There is some controversy whether Gd should be described as truly "ferromagnetic" at room temperatures, but it can indeed be magnetized. (See article by Coey et al below). Its transition temperature (Curie point) is about 19°C (66°F).
In addition to the powerful magnetic moment created by its 7 unpaired electrons, the Gd+3 ion also has a relatively long electron-spin relaxation time (τs ≈ 10−9 sec) at clinically relevant field strengths. This results from the symmetry of the Gd(III) S-state, creating a more hospitable environment for T1-relaxation than other lanthanide ions.
Other transition metal ions commonly considered as MR contrast agents include 24Cr+3, 25Mn+2, 26Fe+3, and 29Cu+2. These ions have 3, 5, 5, and 1 unpaired electron in their 3d shells. The two strongest of these (25Mn+2 and 26Fe+3) have magnetic moments of approximately 5.9 Bohr magnetons, compared to 64Gd+3 at 7.9.
Two other lanthanide ions, 63Eu+3 and 66Dy+3, have 6 and 5 unpaired electrons in their 4f shells, but again come up short compared to 64Gd+3. Due to asymmetry of their electronic S-states, these lanthanides have electron spin relaxation times that are too short for effective T1 relaxation. They are used experimentally as T2 or T2* relaxation contrast agents.
Coey JMD, Skumryev V, Gallagher K. Is gadolinium really ferromagnetic? Nature 1999; 401:35-36. "Gadolinium". Wikipedia, the Free Encyclopedia.
Lauffer RB: Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev 1987; 87:901-927.
Moeller T. Periodicity and the lanthanides and actinides. J Chem Educ 1970; 47:417-423.
Weinmann H-J, Brasch RC, Press W-R, Wesbey GE. Characteristics of Gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roengenol 1984; 142:619-624.
What are the causes of T1 and T2 relaxation?
What is magnetic susceptibility?
How does gadolinium cause relaxation?