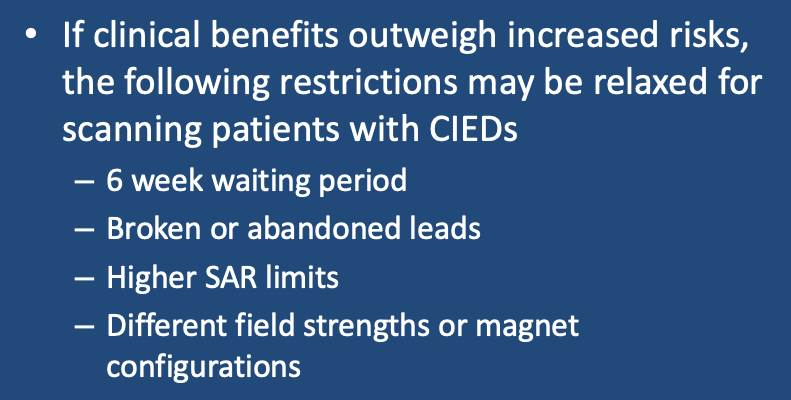
From time to time we encounter a patient that clinically would benefit from an MRI scan yet does not meet all the strict conditions listed in the previous two Q&As. As all treatments in medicine involve benefit vs risk, it is worth considering how stringent one should be concerning relative contraindications.
Recent Implants (< 6 weeks)
Existing guidelines recommend waiting 6 weeks after implantation to allow the electrode lead tips to acquire a fibrous sheath and thereby secure purchase in the myocardium. To me, this restriction is reminiscent of the 6-week MRI delay once required for cardiac stents that has long since been rescinded. Support for scanning patients with pacemakers less than 6 weeks implanted can be found in the papers by Friedman (2013) and Russo (2017) below. I personally have no problem recommending MRI immediately after implantation or anytime during this 6 week window, provided their lead voltages and impedances have been stable. (Admittedly, I might be a little more cautious for leadless pacemakers, as they could become dangerous emboli if dislodged).
Broken or Abandoned Intravascular/Intracardiac Leads
|
The primary concern here is lead heating, resulting in endocardial burns, secondarily induced arrhythmias, damage to adjacent leads, and changes in lead capture and sensing parameters. The recent studies by Higgins (2014) Schaller (2021) and Greenhill (2024) give some comfort that such patients can be safely scanned at 1.5T. If you do choose to scan such patients, try to keep the average SAR ≤ 1.5 W/kg. Note that these restrictions do not apply to retained temporary epicardial leads that may be safely scanned (see separate Q&A).
|
Mismatched Leads and Generator
Occasionally a patient will present with a CIED rendered non-conditional because of a mismatch between manufacturers of the lead(s) and generator. This most commonly occurs when older generators are replaced with a new device and the original wires are left in place. Although little published literature exists concerning this configuration, a recent study by Okasha et al. found no adverse events in 35 patients studied at 1.5T.
Higher SAR Limits
Notwithstanding my comments in the previous section, many authorities believe that higher SAR limits (such as those applicable to First Level Controlled Operating Mode) are fully acceptable to use with both conditional and non-conditional CIEDs. In small series reported, no adverse events from such higher SAR limits have been observed. Until more research is available, however, I prefer to keep our studies within the (lower SAR) Normal Operating Mode.
Different Field Strengths and Magnet Configurations
A common question is whether non-conditional CIEDs can be scanned at 3.0T. It is true that a number of MR-Conditional CIEDs have been approved for 3.0T. Data is largely lacking for non-Conditional CIED at 3.0T, so why risk it? Let's face it, 3.0T is great but nearly every diagnosis usually be made very well on a well-tuned 1.5T scanner.
Whole-body scanners operating below 1.5T are mostly non-cylindrical in design with vertical magnetic fields. As even MR-Conditional CIEDs are not approved in this scenario and very little research exists, it would be prudent to avoid such scanners and locate a suitable wide-bore 1.5T system. Small dedicated head and extremity scanners, however, whose fields are relatively confined, are generally safe to use with all CIEDs.
Whole-body scanners operating below 1.5T are mostly non-cylindrical in design with vertical magnetic fields. As even MR-Conditional CIEDs are not approved in this scenario and very little research exists, it would be prudent to avoid such scanners and locate a suitable wide-bore 1.5T system. Small dedicated head and extremity scanners, however, whose fields are relatively confined, are generally safe to use with all CIEDs.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Friedman HL, Acker N, Dalzell C, et al. Magnetic resonance imaging in patients with recently implanted pacemakers. Pacing Clin Electrophysiol 2013; 36:1090–1095.
Greenhill M, Rangan P, Su W, et al. MRI in patients with cardiovascular implantable electronic devices and fractured or abandoned leads. Radiology: Cardiothoracic Imaging 2024; 6(3):3230404. [DOI LINK]
Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol 2014;37:1284–1290.
Langman DA, Goldberg IB, Finn JP, Ennis DB. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging 2011; 33:426-431. [DOI LINK]
Mattei E, Triventi M, Calcagnini G, et al. Complexity of MRI induced heating on metallic leads: Experimental measurements of 374 configurations. BioMedical Engineering OnLine 2008, 7:11. [DIRECT LINK]
Okasha O, Saeed IM, Gupta SK. Outcome of MRI in patients with nonconditional devices with mismatch between manufacturer of leads and generator. Radiology: Cardiothoracic Imaging 2022; 4(3):e220014. [DOI LINK]
Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017; 376:755–764.
Schaller RD, Bruckner T, Riley MP, et al. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiology 2021; 6:549-556. [DOI LINK]
Friedman HL, Acker N, Dalzell C, et al. Magnetic resonance imaging in patients with recently implanted pacemakers. Pacing Clin Electrophysiol 2013; 36:1090–1095.
Greenhill M, Rangan P, Su W, et al. MRI in patients with cardiovascular implantable electronic devices and fractured or abandoned leads. Radiology: Cardiothoracic Imaging 2024; 6(3):3230404. [DOI LINK]
Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol 2014;37:1284–1290.
Langman DA, Goldberg IB, Finn JP, Ennis DB. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging 2011; 33:426-431. [DOI LINK]
Mattei E, Triventi M, Calcagnini G, et al. Complexity of MRI induced heating on metallic leads: Experimental measurements of 374 configurations. BioMedical Engineering OnLine 2008, 7:11. [DIRECT LINK]
Okasha O, Saeed IM, Gupta SK. Outcome of MRI in patients with nonconditional devices with mismatch between manufacturer of leads and generator. Radiology: Cardiothoracic Imaging 2022; 4(3):e220014. [DOI LINK]
Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017; 376:755–764.
Schaller RD, Bruckner T, Riley MP, et al. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiology 2021; 6:549-556. [DOI LINK]
Related Questions
What precautions must be taken to scan patients with older/legacy/non-conditional pacemakers?
What is SAR?
What is meant by the term "operating mode" of an MR scanner?
What about epicardial CIED's and their abandoned leads?
What precautions must be taken to scan patients with older/legacy/non-conditional pacemakers?
What is SAR?
What is meant by the term "operating mode" of an MR scanner?
What about epicardial CIED's and their abandoned leads?