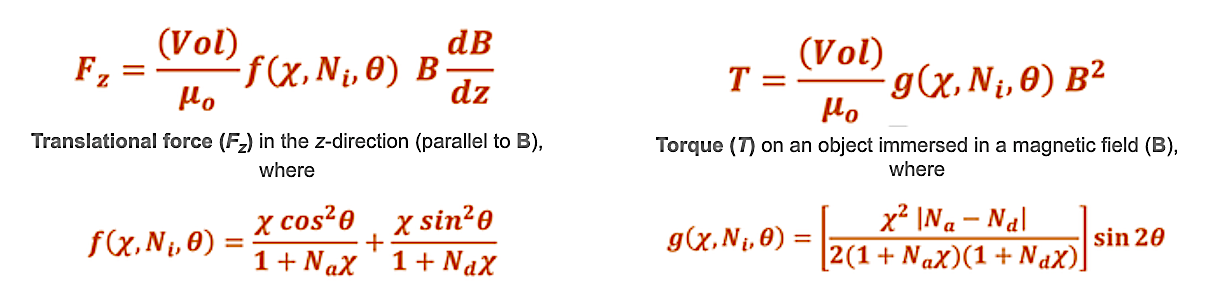Equations describing the magnetic translational force (Fz) and magnetic rotational force/torque (T) on an ellipsoidal metallic object have been developed in a prior Q&A.
Here f(•) and g(•) are functions that depend on the object's intrinsic susceptibility (χ), shape-dependent demagnetizing factors (Na and Nd), and angle the object makes with the external magnetic field (θ). The term μo ≈ 4π x 10−7 N/A² is the magnetic constant, also known as the permeability of free space. For paramagnetic and weakly ferromagnetic materials with χ < 1, f(•) = χ and g(•) = ¼ χ² sin2θ. Further modifications of these equations are needed for materials that reach magnetic saturation or are permanently magnetized.

The American Society for Testing and Materials (ASTM) International, in their core document (F2503), created the categories “MR Safe”, “MR Unsafe”, and “MR Conditional” and developed the familiar icons associated with each. The ASTM has also proposed methods for measuring magnetic displacement force (F2052) and torque (F2213), standards incorporated into a more comprehensive publication by the International Standards Organization (ISO/TS:10974).
ASTM Method for Measuring Translational Magnetic Force
|
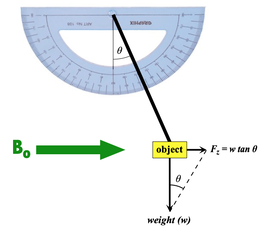
As shown in the diagram, the object in question is suspended by a string near the edge of the scanner bore (where the spatial gradient product Bo•∇Bo is largest). The angular displacement from vertical is then measured, and the translational force along the z-axis (along the direction of the main magnetic field) based on the weight of the object can then be calculated. If the deflection angle is less than 45º, the magnetic force of the object is less than that of gravity, which ASTM considers conditionally safe for this parameter.
|
Even though the magnetic translational force on an implant may exceed that due to gravity, the implant may still be acceptable depending on the mechanical properties of nearby tissues and how tightly the implant is attached in the body. For example, a highly ferromagnetic orthopedic implant may still be conditionally safe provided it is firmly adhered to bone.
For large deflection angles, small errors in angle measurement can produce large errors in the calculated translational force. In this scenario it is common to attach small lead weights to the center of mass of the object to reduce the deflection angle to a more desirable range (25°−65°). For tiny objects like clips, multiple duplicates taped together may be used.
The ASTM method does not consider the shape of the object or the configuration in which it hangs. For example, an elongated object in a field below magnetic saturation will experience a stronger force when aligned with Bo than when perpendicular. Likewise, the magnetic torque on the object is not considered, but this will affect the angle of suspension.
The ASTM method does not consider the shape of the object or the configuration in which it hangs. For example, an elongated object in a field below magnetic saturation will experience a stronger force when aligned with Bo than when perpendicular. Likewise, the magnetic torque on the object is not considered, but this will affect the angle of suspension.
ASTM Methods for Measuring Magnetic Torque
The ASTM offers 5 different methods for measuring magnetic torque on an implant, ranging from simple/qualitative to complex/quantitative. The first three methods (Calculation, Surface, and Suspension) are suitable for objects demonstrating <45° displacements in the translational force test (and which are therefore expected to demonstrate only minor torques. The last two methods (Pulley and Torsion Spring) require more sophisticated setup but are quantitative.
The Calculation Based on Magnetically Induced Force Method is just what the name implies − using the Fz value measured from the Translational Test (F2052) above to set upper limits on the expected torque. The maximum torque, Tmax, is calculated to be
If the magnetic saturation value (Bsat) of the implant material is unknown then the value 2.2T for pure iron is used.
In the Low Friction Surface Method, the propensity of an object to reorient itself with respect to the magnetic field on a smooth plane is noted. This is an all-or-none test. If the object does not move when the plane is rotated, then the torque is considered small, being less than the frictional force times the length of the object. If the object moves, however, then a more sophisticated test quantitative estimate of torque is required.
In the Suspension Method, the implant is suspended from a string a magnet isocenter (where the Bo field is maximal and spatial gradients are zero). After manually twisting the object out of alignment with the field, notation is made concerning the degree to which the object seeks to realign. A subjective assessment using a 5-point scale is often employed (0 = no motion, 2 = moderate, implant aligns gradually with magnetic field, and 4 = very strong torque demonstrating "rapid and forceful" field alignment.)
|
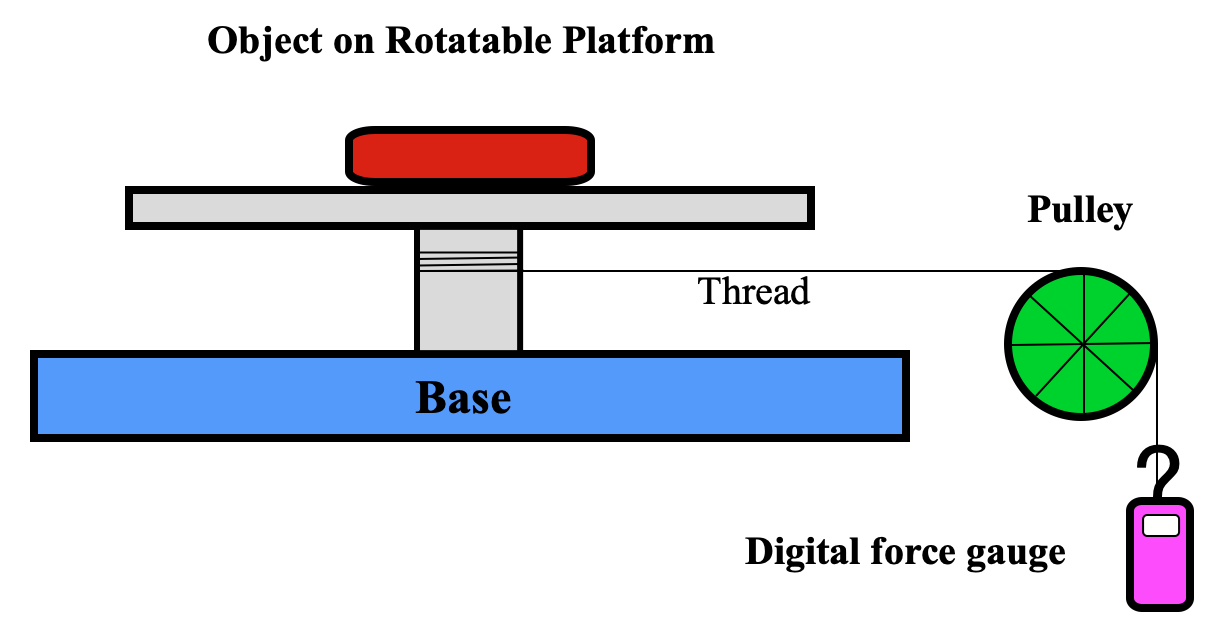
In the Pulley Method, the implant is placed on a platform in near magnet isocenter which is free to rotate via a string and low-friction pulley apparatus. The string is pulled and the object rotated by 360° with the maximum force Fm noted. The object is removed and the rotation repeated to determine the maximum frictional force (Ff) of the pulley system alone. The Torque (T) then equals R(Fm−Ff) where R is the radius.
|
|
The final technique, the Torsion Spring Method, was the only torque measurement standard ASTM recognized up until the current (2017) edition. This method thus has a long history and is considered by many to be the most accurate for implants where an appreciable torque is expected. As shown in the illustration left, the implant is secured by strong tape to a tray suspended between two calibrated torsion springs (blue canisters) and placed at magnet isocenter. The apparatus is then rotated in 10° increments using a turning gear (not pictured) over a full 360° rotation and the maximum deflection angle (Δφ) recorded. The magnetically induced torque (T) equals kΔφ, where k is the torsional spring constant. The calibrated spring system thus allows a numerical value for torque to be obtained, in units such as N-m. |
If the maximal torque recorded by either the Pulley Method or Torsion Spring Method is less than the longest dimension of the object times its weight, then the magnetically induced torque is less than the worst case torque due to gravity. The ASTM then considers the implant conditionally safe on the basis of torque. As with translational forces, objects with torques exceeding the gravity limit may still be acceptable depending on the mechanical properties of nearby tissues and the tightness of the implant's attachment.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
ASTM F2503-20, Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2020, www.astm.org
ASTM F2052-15, Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2015, www.astm.org
ASTM F2213-17, Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2017, www.astm.org
ISO/TS 10974:2018. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. International Standards Organization, Geneva, 2018.
US Food and Drug Administration. Testing and labeling medical devices for safety in the magnetic resonance (MR) environment. Draft Guidance for Industry and Food and Drug Administration Staff. Final version 20 May 2021.
Woods TO. Standards for medical devices in MRI: present and future. J Magn Reson Imaging 2007; 26:1186-1189. [DOI Link]
ASTM F2503-20, Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2020, www.astm.org
ASTM F2052-15, Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2015, www.astm.org
ASTM F2213-17, Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment, ASTM International, West Conshohocken, PA, 2017, www.astm.org
ISO/TS 10974:2018. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. International Standards Organization, Geneva, 2018.
US Food and Drug Administration. Testing and labeling medical devices for safety in the magnetic resonance (MR) environment. Draft Guidance for Industry and Food and Drug Administration Staff. Final version 20 May 2021.
Woods TO. Standards for medical devices in MRI: present and future. J Magn Reson Imaging 2007; 26:1186-1189. [DOI Link]
Related Questions
What are the risks of passive vs active implants in MRI?
How do you calculate the magnetic force pulling a piece of metal toward the scanner?
What are the risks of passive vs active implants in MRI?
How do you calculate the magnetic force pulling a piece of metal toward the scanner?