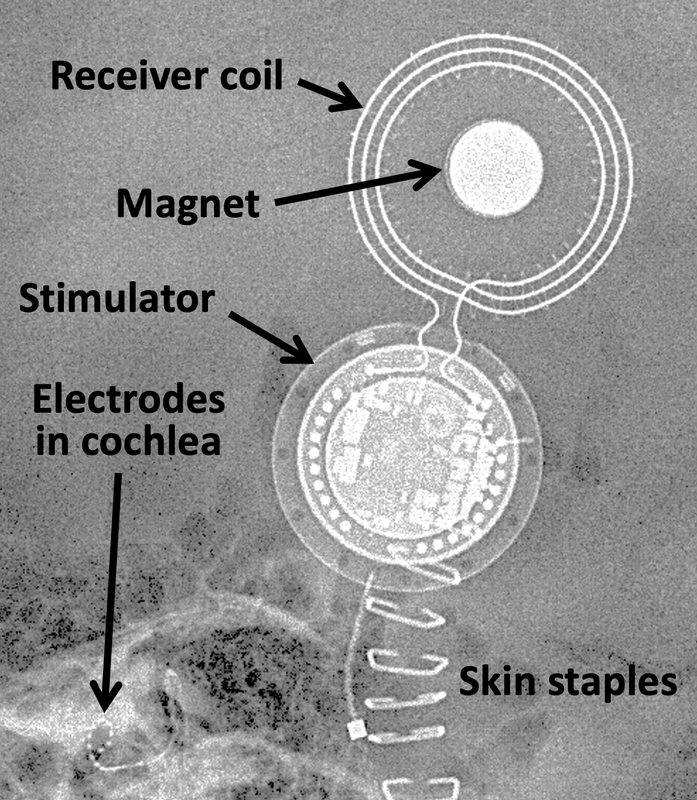
A cochlear implant (CI) is a small, surgically implanted electronic device that directly stimulates the cochlear (auditory) nerve to provide a sense of sound to patients who are profoundly deaf or severely hearing impaired. As shown in the diagram, the typical CI consists of an external microphone and processor that converts sound into electrical impulses. These impulses then travel to an external transmitter that electromagnetically couples with coils on an adjacent subcutaneous receiver. Both the receiver and transmitter contain small permanent magnets allowing them to remain closely apposed during use. The stimulator portion of the subcutaneous implant amplifies and further modifies the sound impulses, sending them down a wire that enters the cochlea through the round window. Signals in the coiled cochlear wire stimulate the cochlear nerve, thus creating a sense of hearing for the patient.
|
|
The receiver/stimulator circuitry is housed in a shell of silicone, titanium, or ceramic and the electrode wires are relatively short. Thus there is no substantial concern about implant or wire heating for most CI's at fields ≤3.0T using normal operating mode. The major concerns all relate to the presence of the magnet in the receiver component. These include magnet movement, demagnetization, and imaging artifacts.
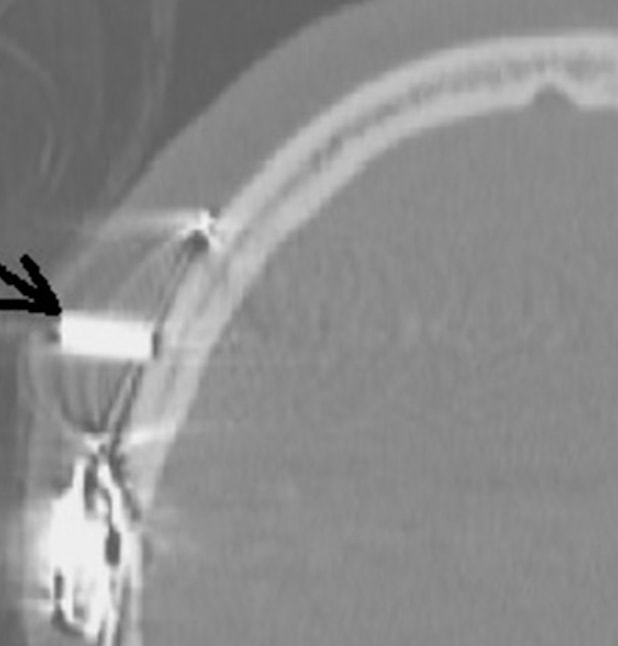
Pain from magnet movement occurs in a substantial fraction of patients. It may be minimized by wrapping the head with a tight-fitting elastic bandage prior to entering the scanner. In some cases the internal magnet must be removed and replaced as a condition of scanning. (This entails simple outpatient surgery under local anesthesia). Magnet dislocation can also occur as in the example shown above left. The patient's head should ideally remain straightly aligned with the bore of the scanner; demagnetization or reversal of magnetization of the implant may occur if the head is tilted more than 30º.
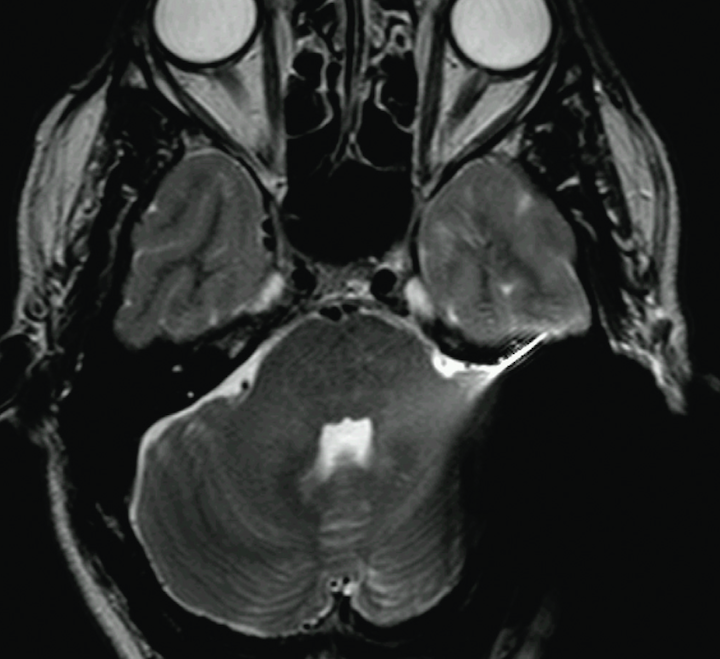
Significant susceptibility artifacts from the implant are unavoidable if the magnet is present. The effect is greater at 3.0T than at 1.5T and worse with GRE than SE sequences. Usually the entire temporal bone and a moderate portion of the regional brain is obscured by these artifacts.
|
About a half-dozen companies manufacture various forms of cochlear prostheses worldwide, the three largest (accounting for 95% of the market) being Cochlear Ltd (Lane Cove, NSW, Australia), MED-EL GmbH (Innsbruck, Austria), and Advanced Bionics Corp (Sylmar, CA, USA). With just a few exceptions, nearly all of the current production models are MR Conditional. Of course, the first "condition" is to remove all external components (microphone, processor, and transmitter) before entering the MRI room! And the second step is to absolutely confirm the exact brand and model number from the patient's implant information card or reliable medical record source.
With so many different implants and their ever-changing designs, it is important to check the manufacturer's web site in each case before scanning a patient. The "Conditions" vary significantly (even from country to country) and must be followed exactly. Just because an implant has conditional approval at 1.5T, for example, does not mean that safe scanning at 0.5 or 1.0T is assured. Other restrictions may include closed vs open bore scanning, maximum allowable SAR, maximum spatial gradient field, optimal patient positioning, use of localized transmit coils, requirements for head wrapping, use of parallel transmit body coils, minimum allowable bone thickness beneath the implant site, time since implantation (some require 6-month waiting period), and whether magnet removal is merely recommended or strictly required prior to imaging.
Cochlear implants were first developed in the 1960s and 1970s with commercial models appearing in the 1980s. A small number of MR-compatible devices were just becoming available in the mid-1990's, but for all practical purposes most implants before 2005-2010 should be considered MR Unsafe. Specific named MR Unsafe devices from this period that may potentially be encountered in clinical practice include the House and Vienna implants (3M), DX-10 (Neurolec), pre-2004 Nucleus models without removable magnets (Cochlear), and Clarion models 1.0, 1.2, and CII Bionic Ear (Advanced Bionics).
 Electrode arrays for cochlear and brainstem implants respectively
Electrode arrays for cochlear and brainstem implants respectively
If the cochlea is severely damaged or the cochlear nerve involved by tumor, the basic CI device (with some modifications) can be used to stimulate the brainstem directly. The resultant apparatus is then known as a brainstem auditory implant (BAI). For this application the distal wire must terminate in a paddle-like electrode grid rather than the half-banded linear array use for standard cochlear stimulation. The paddle is surgically implanted against the lateral pons to stimulate the cochlear nucleus. The same safety issues apply equally to both BAI's and CI's.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Azadarmaki R, Tubbs R, Chen DA, Shellock FG. MRI information for commonly used otologic implants: review and update. Otolaryngol Head Neck Surg 2014;150:512-9. [DOI LINK]
Bawazeer N, Vuong H, Riehm S, et al. Magnetic resonance imaging after cochlear implants. J Otol 2019; 14:22-25. [DOI LINK]
Cochlear Ltd. Cochlear Nucleus Implants. Magnetic Resonance Imaging (MRI) Guidelines. 2020.
Eshraghi AA, Nazarian R, Telischi FF, et al. The cochlear implant: historical aspects and future prospects. Anat Rec 2012; 295:1967-1980. [DOI LINK]
Majdani O, Leitung M, Rau T, et al. Demagnetization of cochlear implants and temperature changes in 3.0T MRI environment. Otolaryngol Head Neck Surg 2008;139:833-839. [DOI Link]
MED-EL Hearing Implants: MRI Safety Status Overview. MED-EL GmbH (Innsbruck, Austria), 2019. (for newest safety updates visit www.medel.com/isi)
Shew M, Wichova H, Lin J, et al. Magnetic resonance imaging with cochleae implants and auditory brainstem implants: are we truly practicing MRI safety? Laryngoscope 2019; 129:482-489. [DOI Link]
Srinivasan R, So CW, Amin N, Jaikaransingh D, et al. A review of the safety of MRI in cochlear implant patients with retained magnets. Clin Radiol 2019; 74:972.e9-972.e16. [DOI Link]
Todt I, Guerkov R, Gehl HB, Sudhoff H. Comparison of cochlear implant magnets and their MRI artifact size. BioMed Res International 2020; 5086291:1-8 [DOI LINK]
Azadarmaki R, Tubbs R, Chen DA, Shellock FG. MRI information for commonly used otologic implants: review and update. Otolaryngol Head Neck Surg 2014;150:512-9. [DOI LINK]
Bawazeer N, Vuong H, Riehm S, et al. Magnetic resonance imaging after cochlear implants. J Otol 2019; 14:22-25. [DOI LINK]
Cochlear Ltd. Cochlear Nucleus Implants. Magnetic Resonance Imaging (MRI) Guidelines. 2020.
Eshraghi AA, Nazarian R, Telischi FF, et al. The cochlear implant: historical aspects and future prospects. Anat Rec 2012; 295:1967-1980. [DOI LINK]
Majdani O, Leitung M, Rau T, et al. Demagnetization of cochlear implants and temperature changes in 3.0T MRI environment. Otolaryngol Head Neck Surg 2008;139:833-839. [DOI Link]
MED-EL Hearing Implants: MRI Safety Status Overview. MED-EL GmbH (Innsbruck, Austria), 2019. (for newest safety updates visit www.medel.com/isi)
Shew M, Wichova H, Lin J, et al. Magnetic resonance imaging with cochleae implants and auditory brainstem implants: are we truly practicing MRI safety? Laryngoscope 2019; 129:482-489. [DOI Link]
Srinivasan R, So CW, Amin N, Jaikaransingh D, et al. A review of the safety of MRI in cochlear implant patients with retained magnets. Clin Radiol 2019; 74:972.e9-972.e16. [DOI Link]
Todt I, Guerkov R, Gehl HB, Sudhoff H. Comparison of cochlear implant magnets and their MRI artifact size. BioMed Res International 2020; 5086291:1-8 [DOI LINK]
Related Questions
What other types of otologic devices should be viewed with concern for MRI?
Are bone conduction implants safer than cochlear implants for MRI?
What other types of otologic devices should be viewed with concern for MRI?
Are bone conduction implants safer than cochlear implants for MRI?