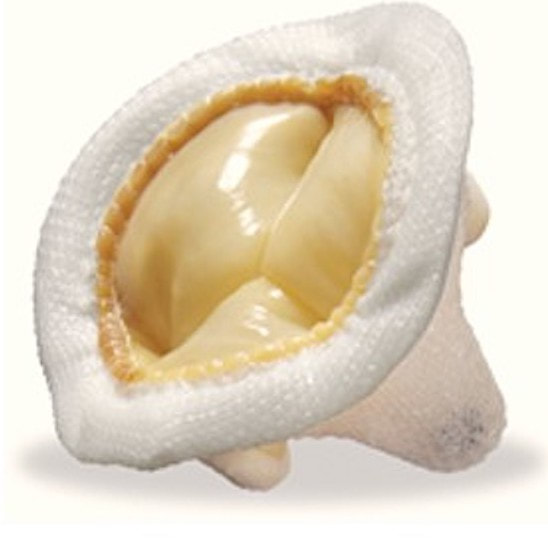
- Bioprosthetic valves are either homografts (made from human tissue) or xenografts (porcine, bovine, equine). Some have no metallic components and are MR Safe at all field strengths. (Their visibility on x-ray is due to admixture of a small amount of barium sulfate to the silicone structure). Others, especially transcatheter ones, have support rings or stents containing titanium, tungsten, or non-ferromagnetic alloys, making them MR Conditional. Because the lifespan of bioprosthetic valves is only 10 to 20 years, they are typically implanted in older patients.
- Mechanical valves all contain metal (titanium or an alloy) at least in their support structure. Currently available valves have either one or two tilting disks. Older mechanical valves (such as Björk-Shiley or caged-ball models, including some made of stainless steel) may rarely be encountered, but like their modern counterparts are considered MR Conditional.
|
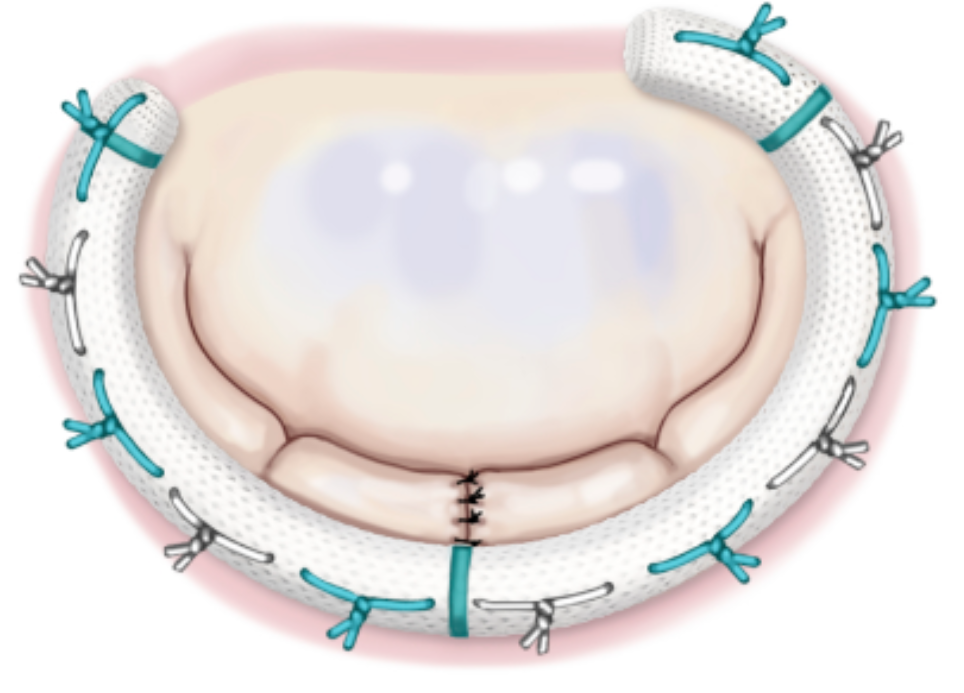
Most common surgically implanted annuloplasty devices have no metallic components and are MR Safe at all field strengths. However, the popular Carpentier-Edwards (Edwards LifeSciences) series and several others are composed of titanium alloys and are MR Conditional.
|

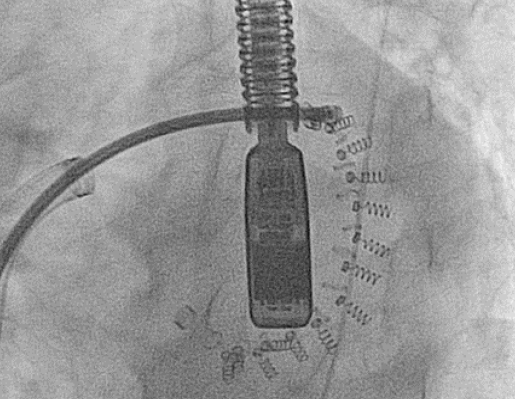
"MR Conditional" mitral annuloplasty device on chest x-ray. (Case courtesy of Dr Matt A. Morgan, Radiopaedia.org.
|
- Carillon Mitral Contour System® (Cardiac Dimensions) is a flexible wire titanium-alloy device that is placed in the coronary sinus to wrap around and reshape the mitral valve indirectly.
- Cardioband® (Edwards LifeSciences) is a direct annuloplasty method wherein an adjustable C-shaped Dacron band is deployed via a transseptal approach and anchored to the left atrial side of the mitral annulus.
|
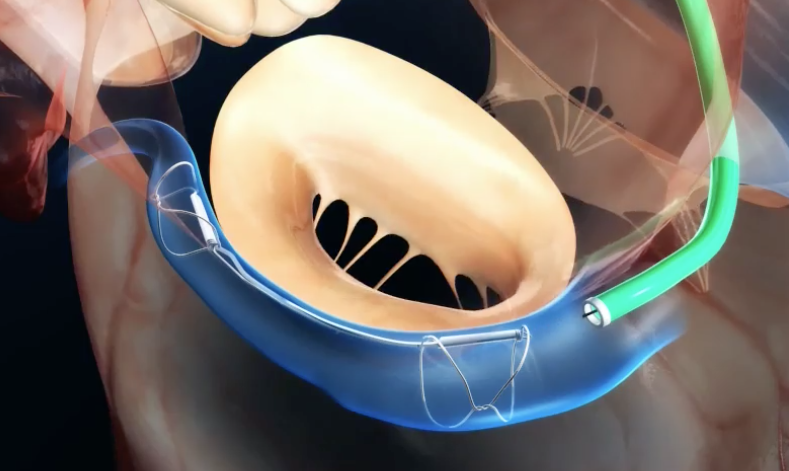
Two very similar MR Conditional transcather mitral valve repair systems are also commercially available, indicated for high risk patients who cannot tolerate an open surgical procedure — the MitraClip® (Abbott) and the PASCAL® Repair System (Edwards LIfeSciences). The clips are made of Nitinol®.
After entering the right heart by a venous approach, the intra-atrial septum is penetrated by the guide catheter and advanced so its tip lies in the left ventricle just below the margins of the mitral valves. A small clip then captures each valve leaflet pulling them together, in theory thus reducing mitral regurgitation. |
Advanced Discussion (show/hide)»
Pre-6000 series Starr-Edwards valves were once considered contraindicated for MR imaging, but this prohibition has now been removed. In any case, because most of these valves were implanted over 40 years ago, few, if any, patients are still alive who received them.
At one time worries existed that an electromagnetic phenomenon known as the "Lenz effect" could inhibit the opening and closing of valves (such as the Björk-Shiley) made of metal disks or leaflets. This theoretical concern was not born out experimentally, so all-metal valves remain safe to scan at fields at least up to 3T.
Carpentier-Edwards annuloplasty rings, Models 4400 and 4500, marketed from 1980 to 1983, were made of stainless steel. The manufacturer is unwilling to provide an MR safety statement, so these must be considered "MR Unsafe." It is highly unlikely, however, that anyone with mitral valve disease requiring angioplasty 40 years ago is still alive to be scanned, so I do not worry about the possibility of such a "black swan"event.
Edwards M-B, Draper ERC, Hand JW, et al. Mechanical testing of human cardiac tissue: some implications for MRI safety. J Cardiovasc Magn Reson 2005;7:835-840.
Edwards M-B, Ordidge RJ, Thomas DL, et al. Translational and rotational forces on heart valve prostheses subjected ex vivo to a 4.7 T MR system. J Magn Reson Imaging 2002; 16:653 –659. (testing of 60 valves at 4.7T suggests that these are conditionally safe as well).
Edwards M-B, Ordidge RJ, Hand JW, et al. Assessment of magnetic field (4.7T) induced forces on prosthethic heart valves and annuloplasty rings. J Magn Reson Imaging 2005; 22:311-317. (prior work extended to include more annuloplasty rings) [DOI LINK]
Edwards M-B, Taylor KM, Shellock FG. Prosthetic heart valves: evaluation of magnetic field interactions, heating, and artifacts at 1.5T. J Magn Reson Imaging 2000;12:363-9.
Kuwata S, MAisano F. State of the art of transcatheter mitral annuloplasty: present and future. Cardiac Interventions Today 2018; 12:41-43.
Myers PO, Kalangos A, Panos A. Safety of magnetic resonance imaging in cardiac surgery patients: annuloplasty rings, septal occluders, and transcatheter valves (letter and response). Ann Thor Surg 2012; 93:1019-20. [DOI LINK]
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021: 143:e72-e227. [DOI LINK]
Society for Medical Physics of the Netherlands (NVKF). Guideline use of MRI in patients with implants. Module 1: MRI in patients with a prosthetic heart valve, annuloplasty ring or mitra clip. English version 2021.