BOLD Hemodynamic Response Function (HRF)
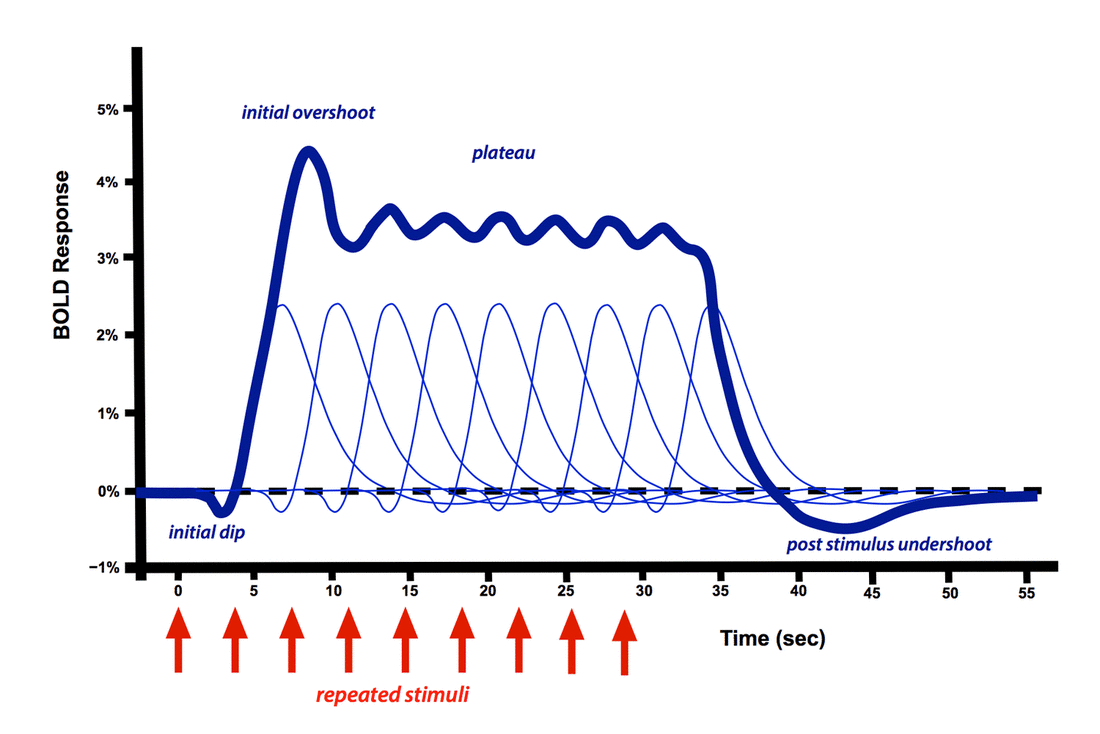
BOLD Hemodynamic Response Function (HRF)following a single brief stimulus
The positive dominant peak constitutes the bulk of the BOLD response, quickly overwhelming the small initial dip. During this phase regional cerebral blood flow increases out of proportion to immediate metabolic needs. The result is that the ratio of oxyhemoglobin to deoxyhemoglobin transiently increases, with increased MR signal. Even with a very brief stimulus, the dominant HRF response is sluggish and delayed, often not occurring until 5-15 seconds later.
|
Multiple repeated stimuli add together in an approximately linear fashion as long as the time between stimuli exceeds 4-5 seconds. When a long train of repeated stimuli are applied, the dominant peak becomes a broad plateau, not dropping off until the stimulation ends. A small initial overshoot may sometimes be observed. Of course, considerable variation of the HRF exists between different subjects, experimental conditions, and brain regions studied.
|
|
|
More details about the HRF and BOLD response are available in the short video (left) starring Martin Lindquist from Johns Hopkins and Tor Wager from the University of Colorado at Boulder. This is part of a much larger and highly recommended video series available at their Principles of fMRI YouTube Channel accessible at this link.
|
Advanced Discussion (show/hide)»
Neurovascular Coupling
Neurovascular coupling refers to the processes by which neuronal activity induce changes in regional blood flow, blood volume, and oxygen extraction. Notwithstanding over a century of considerable research, the principal mechanisms underlying this phenomena are still poorly understood with no scientific consensus yet reached.
Metabolic needs. The brain has high energy needs, using about 20% of the O2 consumed by the entire body. At least half of this energy is involved with synaptic transmission, including reversal of ion fluxes through postsynaptic receptors and recycling of glutamate. The time course of the BOLD response with initial overshoot of blood supply and hyperoxygenation exceeds immediate metabolic needs. This suggests that the late supply of blood may be used to replenish energy supplies to neurons and their associated support cells (astrocytes/pericytes) rather than be involved in their initial response. The supply of glucose, rather than oxygen, may be the most important factor.
Vasoactive substances. Neuronal activity results in the release of numerous ions and small molecules that are vasoactive. These include ions (Ca++, K+, Na+), adenosine, nitrous oxide (NO), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), prostaglandins, glutamate, acetylcholine, and norepinephrine.
Propagated vasodilatation. Signaling mechanisms within vessels themselves mediated by endothelial-derived hyperpolarizing factor (EDHF), prostanoids, and/or nitrous oxide are just now being explored as contributors to the hemodynamic response.
Cellular mediators besides neurons (astrocytes and pericytes). Glial cells outnumber neurons by a factors exceeding 10-fold and comprise about half of total brain volume. They play an important role in cerebrovascular regulation, neurometabolic regulation, neurotransmitter uptake, and quite possibly the BOLD signal itself. Astrocytes, in particular, are receiving considerable scrutiny at present due to their unique position interfacing with neurons and blood vessels. A current popular (but unproven theory) is the "astrocyte-neuron lactate shuttle" which posits that astrocytes supply lactate as an energy substrate to neurons in response to elevated gluatmate levels.
Dissociation of neuronal activity and BOLD
Occasionally the BOLD signal may be dissociated or discordant from neuronal activation. A commonly cited example is the phenomenon of anticipatory vasodilatation in which BOLD signal may increase prior to the arrival of an expected repeated stimulus. Another example is involves administration of the GABAA agonist picrotoxine where increased action potential spiking occurs without change in regional cerebral blood flow.
Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med 1992; 25:390–397.
Blamire AM, Ogawa S, Ugurbil K, et al. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci USA 1992; 89:11069–11073. (first demonstration of the single HRF from a short stimulus versus plateau-type response from much longer stimuli).
Buckner RL. Event-related fMRI and the hemodynamic response. Human Brain Mapping. 1998; 6:373–377. (shows linear response to short-term stimuli)
Chen JJ, Pike GB. Origins of the BOLD post-stimulus undershoot. Neuroimage 2009; 46:559-568.
Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev 2010; 62:233-244.
Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci 2011; 33:577-588. (Astrocytes outnumber of neurons by 10:1 and comprise about half of total brain volume. They play an important role in cerebrovascular regulation, neurometabolic regulation, neurotransmitter uptake, and quite possibly the BOLD fMRI signal)
Fox PT. The coupling controversy. Neuroimage 2012; 62:594-601.
Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 1986; 83:1140-1144. (Famous paper destroying the once-popular myth that CBF and metabolic rate of oxygen consumption were tightly linked).
Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron 2012; 75:762-777. (Good review showing that over half of brain energy is used for synaptic transmission).
Hillman EMC. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 2014; 37:161-181.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412:150-157. (famous paper showing better correlation of BOLD responses with LFPs rather than action potentials)
Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Poststimulus undershoots in cerebral blood flow and BOLD fMRI responses are modulated by poststimulus neuronal activity. Proc Natl Acad Sci USA 2013; 110:13636-41.
Phillips AA, Chan FHN, Zheng MMZ, et al. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2015; Epub http://dx.doi.org/10.1177/0271678X15617954
Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neurosci 2006; 9:569-577. (description of the negative BOLD response)
Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 2008; 457:475-479, corrected 5 Feb 2009. (showed that vasodilation may bring additional arterial blood to cortex locally in anticipation of expected tasks)
Wager,TD, Vazquez A, Hernandez L, Noll DC. Accounting for nonlinear BOLD effects in fMRI: Parameter estimates and a model for prediction in rapid event-related studies. NeuroImage 2005; 25:206–218. (HRFs do not add linearly when events are applied at intervals less than ~2 sec)