Mentor CX2 breast tissue expander
Mentor CX2 breast tissue expander
Tissue expanders are balloon-like devices temporarily placed in the operative bed after mastectomy during the first stage of breast reconstruction. They typically have silicone outer shells and a superficial valve or port. Saline is injected at regular intervals over several months to stretch the skin and overlying muscles. Once they have attained their desired size, the expanders are removed and replaced with permanent saline or silicone-gel breast prostheses (all of which are MR Safe). Breast tissue expanders in most common use today are manufactured by Mentor (Johnson & Johnson), Allergan, and Sientra.
For the last 25 years, most tissue expanders have included a MAGNA-SITE® port for injection. This port consists of a central Neodymium magnet set into the base of a titanium plate with a slight rim. The plate serves as puncture-proof needle guard. The injection site can be easily located using a hand-held magnetic finder. Because the expander is usually placed deep to the pectoralis muscle, the port cannot be identified by palpation.
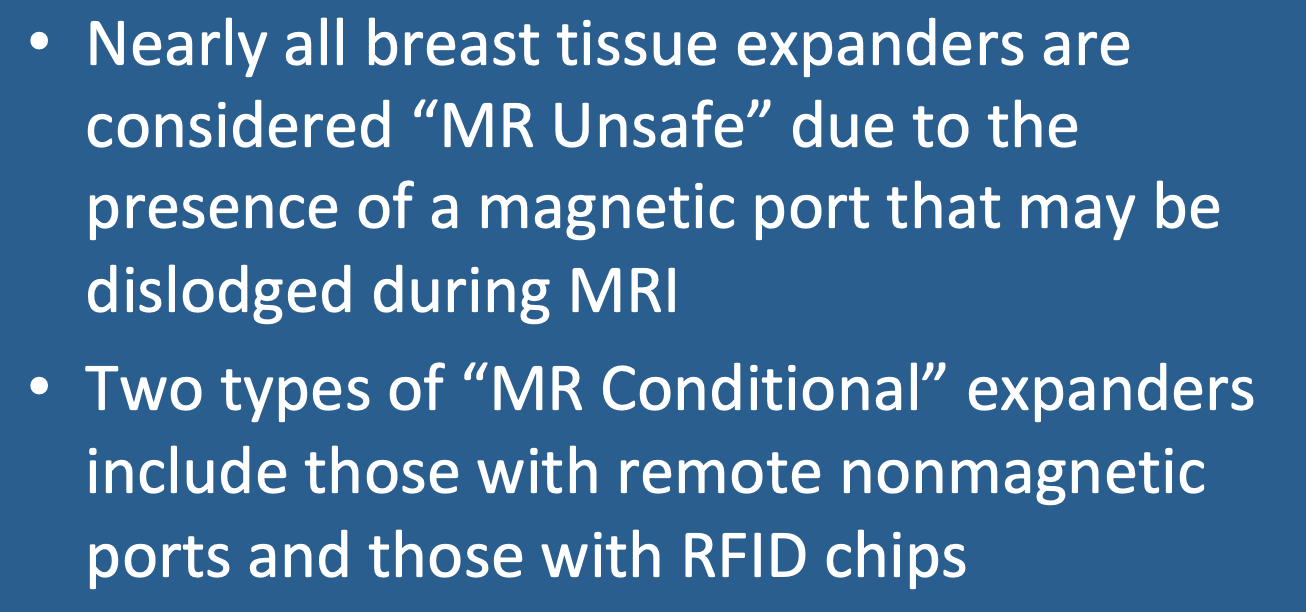
Notwithstanding its clinical utility, the presence of a magnetic injection port is problematic concerning MR safety. Several cases have been reported where patients have experienced localized heating or discomfort or have actually had their ports dislodged during MRI. Although the number of such incidents is very small, every major manufacturer of tissue expanders with magnetic ports have declared these devices to be MR Unsafe. Notwithstanding these restrictions, radiologists at Cornell have performed 1.5T MRI on over 200 patients with magnetic ports without any complications or problems.
Notwithstanding its clinical utility, the presence of a magnetic injection port is problematic concerning MR safety. Several cases have been reported where patients have experienced localized heating or discomfort or have actually had their ports dislodged during MRI. Although the number of such incidents is very small, every major manufacturer of tissue expanders with magnetic ports have declared these devices to be MR Unsafe. Notwithstanding these restrictions, radiologists at Cornell have performed 1.5T MRI on over 200 patients with magnetic ports without any complications or problems.
|
A second MR Unsafe type of tissue expander is the unique AeroForm System (AirXpanders®). Instead of using injectable saline, the AeroForm contains a stainless-steel canister filled with pressurized CO2 gas that expands the implant. An antenna and electronic circuitry is also present to receive the signal transmitted from a hand-held controller that triggers incremental gas release.
|
Fortunately, a few tissue expanders exist that do not contain magnets and can be used in the MR environment: options exist for tissue expanders that are MR Conditional. These include:
- Expanders with remote nonferromagnetic ports. Examples include the Integra (PMT Corp) and the Becker (Mentor/J&J). The remote port (made of titanium, stainless steel, or plastic) is placed subcutaneously where it can be easily palpated and far away from the implant itself.
- Expanders with Radiofrequency ID port locator. Newly CE-marked but not yet FDA-approved, the Motiva Flora Tissue Expander (Establishment Labs) contains an RFID chip instead of a magnet to identify the proper site for injection. It also utilizes a hard plastic instead of a titanium shell to prevent needle punctures.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Bayasgalan M, Munhoz AM, Shellock FG. Breast tissue expander with radiofrequency identification port: assessment of MRI issues. AJR Am J Roengenol 2020; 215:159-164. [DOI LINK]
Bertozzi N, Pesce M, Santi P, Raposio E. Tissue expansion for breast reconstruction: methods and techniques. Ann Med Surg 2017; 21:34-44. [DOI LINK]
Dibbs R, Culo B, Tandon R, et al. Reconsidering the “MR Unsafe” breast tissue expander with magnetic infusion port: a case report and literature review. Arch Plast Surg 2019; 46:375-380. [DOI LINK]
Duffy FJ Jr, May JW Jr. Tissue expanders and magnetic resonance imaging: the “hot” breast implant. Ann Plast Surg 1995;35:647-9. (burning sensation) [DOI LINK]
Dziemianowicz E, Gardner SJ, Snyder KC, et al. Modeling AeroForm tissue expander for postmastectomy radiation therapy. J Appl Clin Med Phys 2019; 20:87-97. [DOI LINK] (Image above from this paper under CC BY)
Stueber K. A complication of tissue expander breast reconstruction. Plast Reconstr Surg 1997;99:1464–1465. (magnetic port dislodgement) [DOI LINK]
Yoon J, Xie Y, Heins D, Zhang R. Modeling of the metallic port in breast tissue expanders for photon radiotherapy. J Appl Clin Med Phys 2018; 19:3:205-214. [DOI LINK]
Zegzula HD, Lee WP. Infusion port dislodgement of bilateral breast tissue expanders after MRI. Ann Plast Surg 2001; 46:46-8. (bilateral dislodgement)
Bayasgalan M, Munhoz AM, Shellock FG. Breast tissue expander with radiofrequency identification port: assessment of MRI issues. AJR Am J Roengenol 2020; 215:159-164. [DOI LINK]
Bertozzi N, Pesce M, Santi P, Raposio E. Tissue expansion for breast reconstruction: methods and techniques. Ann Med Surg 2017; 21:34-44. [DOI LINK]
Dibbs R, Culo B, Tandon R, et al. Reconsidering the “MR Unsafe” breast tissue expander with magnetic infusion port: a case report and literature review. Arch Plast Surg 2019; 46:375-380. [DOI LINK]
Duffy FJ Jr, May JW Jr. Tissue expanders and magnetic resonance imaging: the “hot” breast implant. Ann Plast Surg 1995;35:647-9. (burning sensation) [DOI LINK]
Dziemianowicz E, Gardner SJ, Snyder KC, et al. Modeling AeroForm tissue expander for postmastectomy radiation therapy. J Appl Clin Med Phys 2019; 20:87-97. [DOI LINK] (Image above from this paper under CC BY)
Stueber K. A complication of tissue expander breast reconstruction. Plast Reconstr Surg 1997;99:1464–1465. (magnetic port dislodgement) [DOI LINK]
Yoon J, Xie Y, Heins D, Zhang R. Modeling of the metallic port in breast tissue expanders for photon radiotherapy. J Appl Clin Med Phys 2018; 19:3:205-214. [DOI LINK]
Zegzula HD, Lee WP. Infusion port dislodgement of bilateral breast tissue expanders after MRI. Ann Plast Surg 2001; 46:46-8. (bilateral dislodgement)
Related Questions
Which breast biopsy devices and markers are MRI compatible?
Which breast biopsy devices and markers are MRI compatible?