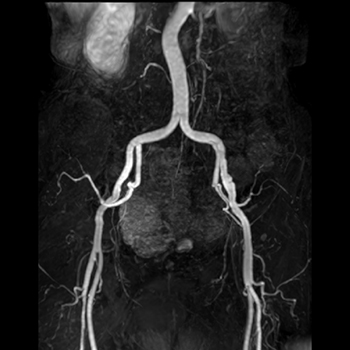
This non-contrast MRA technique is particularly suitable for peripheral angiography. The core sequence is a T2-weighted 3D-fast (turbo) spin-echo accelerated using partial Fourier acquisition. The sequence is cardiac gated either to the EKG or peripheral pulse. Co-registered images are obtained in diastole and systole, and then subtracted to produce an arteriogram.
The idea is straightforward and was first proposed in the 1980s, but has only become practical in the modern era with faster gradients and pulse sequences. During diastole, the signal in both arteries and veins is high reflecting the long T2 of blood. During systole, venous signal remains high but arterial signal drops due to flow-related signal loss. Subtraction of the systolic from the diastolic images results in a pure arterial image.
The idea is straightforward and was first proposed in the 1980s, but has only become practical in the modern era with faster gradients and pulse sequences. During diastole, the signal in both arteries and veins is high reflecting the long T2 of blood. During systole, venous signal remains high but arterial signal drops due to flow-related signal loss. Subtraction of the systolic from the diastolic images results in a pure arterial image.
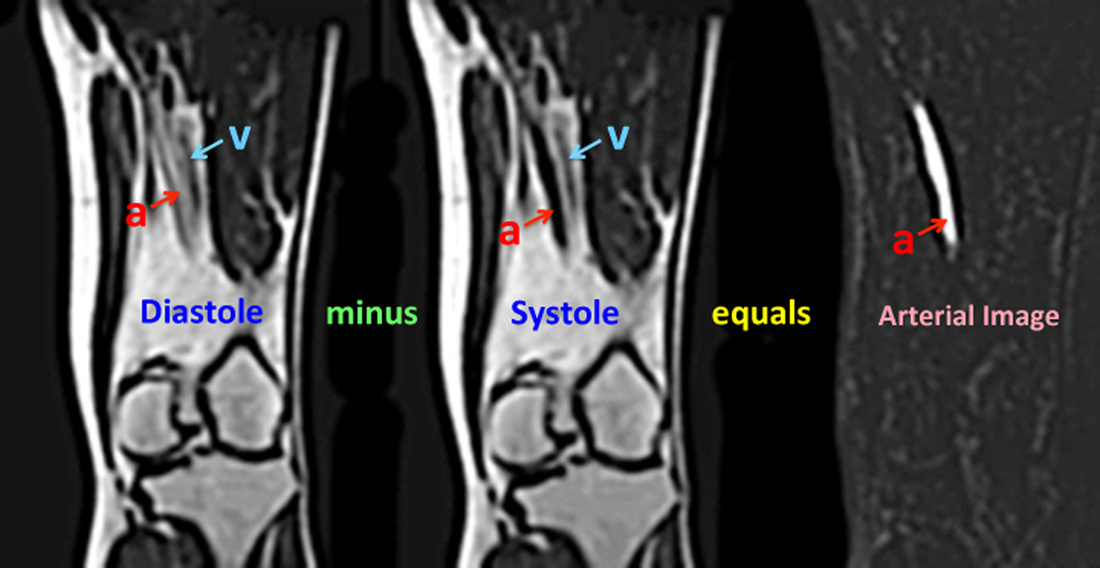
Gated FSE MRA of the distal femoral artery. In diastole (left image), the arterial signal (a) is relatively high, while in systole (middle image) a dark arterial flow void occurs. Intermediate (gray) signal from the adjacent femoral vein (v) remains relatively constant at all phases of the cardiac cycle. Subtracting the systolic from the diastolic image removes the vein as well as most of the soft tissues, providing an arterial image (right).
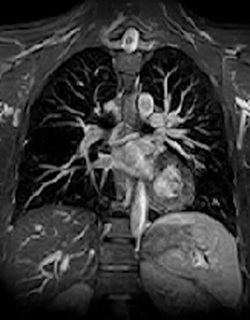
In addition to imaging peripheral arteries, gated 3D-FSE MRA can also be used for imaging the major vessels of the chest, abdomen, and pelvis. If only gross anatomic information about the aorta or major thoracic vessels is needed, a single diastolic image may suffice as nearby venous enhancement will not significantly affect image quality. The technique is useful for non-contrast pulmonary arteriography as the FSE method minimizes susceptibility-induced dephasing at air-vascular interfaces.
As with all other sequences, a vendor-specific nomenclature exists. Perhaps the most elaborate acronym is Siemens' NATIVE SPACE ("Non-contrast MRA of ArTerIes and VEins using Sampling Perfection with Application optimized Contrasts using different flip angle Evolution.") GE's version is called Inhance Deltaflow ("Delta" refers to the fact that a difference image is obtained). Philips uses the acronym TRANCE ("TRiggered Angiography Non-Contrast Enhanced). Hitachi calls their method VASC-FSE ("Veins and Arteries Sans Contrast-Fast Spin Echo"), while Canon uses the acronyms FBI ("Fresh Blood Imaging") and CIA ("Contrast-free Improved Angiography").
The main advantage of 3D-FSE MRA is that it does not require contrast, a property that may be important in patients with renal insufficiency (where receiving gadolinium might place them at risk for nerphogenic systemic fibrosis). If contrast administration is not contraindicated, however, then a contrast-enhanced MRA is generally preferable because of higher image quality and faster imaging times.
A major disadvantage of 3D-FSE, particularly for peripheral MRA, occurs when flow is sluggish or highly stenotic lesions are present. In such situations the pulse wave is damped and the difference in arterial signal between systole and diastole is minimal, resulting in poor arterial contrast on the MRA. Timing of the acquisitions can also be tricky, as the peak systolic wave may arrive in the two extremities at different times. This may require collecting several sets of images at different delay times in different parts of each extremity. In brief, 3D-FSE MRA works great in normal volunteers, but the results may prove unsatisfactory in patients with significant vascular disease.
Other disadvantages of 3D-FSE MRA include: 1) obscuring vessel wall abnormalities such as intramural hematomas and dissections due to the subtraction process; 2) overestimation of stenoses caused by imperfect timing of the triggered acquisitions; 3) gross motion and vascular pulsation artifacts; 4) sensitivity to cardiac arrhythmias; and 5) spatial blurring due to T2-decay during the rapid FSE echo train. The technique is not well suited for carotid imaging because rapid flow in the jugular vein often contaminates the subtraction images. Likewise the gated 3D-FSE method does not work well where orthogonal directions of arterial flow occur, such as between the aorta and renal or mesenteric arteries.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Axel L, Morton D. MR flow imaging by velocity-compensated/uncompensated difference images. J Comput Assist Tomogr 1987; 11:31-34.
Meuli RA, Wedeen VJ, Geller SC, et al. MR gated subtraction angiography: evaluation of lower
extremities. Radiology 1986; 159:411–418. (The original paper describing this technique; it took 20 years for the method to be commonly used, however.)
Miyazaki M, Takai H, Sugiura S, et al. Peripheral MR angiography: separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three dimensional half-Fourier spin-echo imaging. Radiology 2003; 227:890-896.
Axel L, Morton D. MR flow imaging by velocity-compensated/uncompensated difference images. J Comput Assist Tomogr 1987; 11:31-34.
Meuli RA, Wedeen VJ, Geller SC, et al. MR gated subtraction angiography: evaluation of lower
extremities. Radiology 1986; 159:411–418. (The original paper describing this technique; it took 20 years for the method to be commonly used, however.)
Miyazaki M, Takai H, Sugiura S, et al. Peripheral MR angiography: separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three dimensional half-Fourier spin-echo imaging. Radiology 2003; 227:890-896.