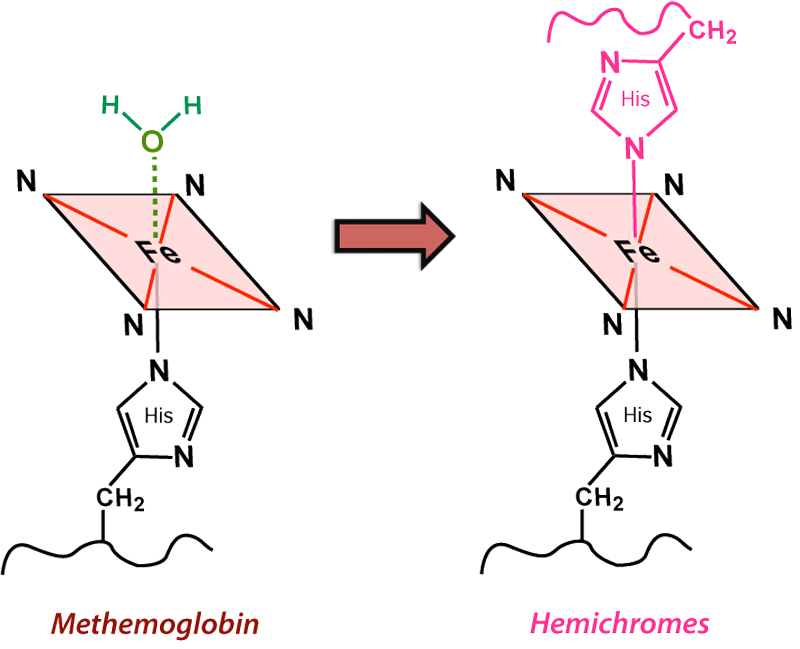
Hemichromes are typically produced by the slow denaturation of methemoglobin. During this process, the H2O molecule at the sixth coordination site is replaced by an amino acid (typically His) from a distal part (E7) of the globin chain. This oxidation process is accompanied by the release of a superoxide (O2−) radical with conversion of iron to a low-spin Fe(III) state that has lost its paramagnetic properties. Hemichromes thus have weak diamagnetism and their MR signal characteristics are dominated by the background material (usually water or residual serum components) within a hematoma cavity.
Finally, the heme units themselves and ultimately Fe dissociate into their individual components. Enzymatic cleavage and recycling of hemoglobin protein fragments occurs, while iron is scavenged by macrophages and glial cells and collected as ferritin and hemosiderin, the subjects of our next Q&A.
Advanced Discussion (show/hide)»
Several chemically distinct hemichromes have been identified depending on what portion of the globin chain comes in to fill the sixth iron coordination site. Some are called "reversible" hemichromes because they may be reconverted back to deoxy-Hb in vitro by treatment with mild reducing agents under anaerobic conditions. It is not clear, however, whether reversibility actually occurs in vivo. For the most part hemichrome formation should be considered an irreversible process and accompanied by relatively severe distortions in the protein tertiary structure.
Another seldom mentioned nonfunctional hemoglobin derivative produced during the denaturation of methemoglobin is sulfhemoglobin. Sulfhemoglobin is a reduced ferrous (Fe+2) compound with a bright green hue having one sulfur atom attached to a β carbon in the porphyrin ring. Like deoxy-Hb it has 4 unpaired electrons and is paramagnetic. It can also weakly bind O2, but with an affinity only one-hundredth that of unmodified Hb.
Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology 1993; 189:15-26.
Gomori JM, Grossman RI. Mechanisms responsible for the MR appearance and evolution of intracranial hemorrhage. Radiographics 1988; 8:427-440.
Pauling L, Coryell CD. The magnetic properties and structure of the hemochromogens and related substance. Proc Natl Acad Sci USA 1936; 22:159-163.
Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cerebral Blood Flow Metabolism 2003; 23:629-652.
What are the different forms of hemoglobin and why do they have different magnetic properties?