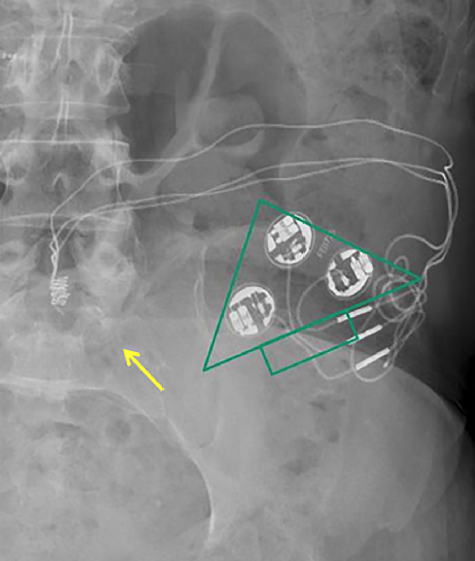
"MR conditional" InterStim® sacral nerve simulator
"MR conditional" InterStim® sacral nerve simulator
Sacral nerve stimulators are an option for patients with refractory lower urinary tract dysfunction and/or fecal incontinence. Neuromodulation devices are made by several manufacturers, the most widely used being Medtronic's InterStim® (pictured right). Like cardiac pacemakers they have a titanium-encased pulse generator with battery subcutaneously planted in the upper buttocks, connected by a thin wire inserted through a sacral foramen (usually S3). The wire terminates as an electrode in the pelvis in the region of the S2-S4 sacral nerves. Sometimes the electrode is placed on the (more distal) dorsal genital or pudendal nerves, which contain more of the S2-S4 direct sensory fibers from the bladder so that motor stimulation in the buttocks is minimized. Some newer devices (e.g., the Axonics® SNM System) have batteries rechargeable by transcutaneous induction.
The Finetech-Brindley Bladder Control System (branded as VOCARE® in the US) has a different design. Implanted in over 3000 patients worldwide since the 1980s, this device treats urinary incontinence in persons with complete spinal cord injuries. The VOCARE® system has both internal and external components. Laminectomies are performed with epidural placement of electrodes along the anterior S2, S3, and S4 roots. The posterior roots are surgically lysed to reduce unwanted sensory feedback contraction of the bladder. The device has no battery but receives power and stimuli transcutaneously by induction between an external transmitter and subcutaneously implanted receivers.
MR Safety Issues
Some older models of sacral nerve neuromodulators are considered MR Unsafe, but most currently implanted models are rated MR Conditional. The exact brand, model name and number must be definitively ascertained and the manufacturer's updated conditions followed exactly.
Regardless of the model and manufacturer, certain common conditions apply to all sacral nerve stimulators. First, the device must be fully functioning and an x-ray obtained if there is any question about the possibility of broken or disconnected leads. The stimulator should be turned off or placed in MRI mode (if available). No external equipment such as transmitters or controllers should be brought into the MR environment.
Advanced Discussion (show/hide)»

Posterior Tibial Nerve Stimulators
Stimulating the tibial nerve??? Isn't that in the leg!?
Initially met with skepticism, electrical stimulation of the posterior tibial nerve is now a scientifically proven treatment for overactive bladder. The exact mechanism is unknown, but peripheral electrostimulation apparently disrupts neural pathways of the lower urinary tract and modulates higher spinal or brainstem reflexes for micturition.
To my knowledge there is only one implantable tibial nerve stimulator system now manufactured, BlueWind RENOVA™ (which has CE mark but not yet FDA approval). Although no longer in production, another tibial nerve stimulator, the Urgent-SQ, may occasionally be encountered. Both devices include a passive receiver element with electrodes implanted near the tibial nerve and require an additional external pulse generator that sends power and stimuli to the receiver transcutaneously.
Stimulating the tibial nerve??? Isn't that in the leg!?
Initially met with skepticism, electrical stimulation of the posterior tibial nerve is now a scientifically proven treatment for overactive bladder. The exact mechanism is unknown, but peripheral electrostimulation apparently disrupts neural pathways of the lower urinary tract and modulates higher spinal or brainstem reflexes for micturition.
To my knowledge there is only one implantable tibial nerve stimulator system now manufactured, BlueWind RENOVA™ (which has CE mark but not yet FDA approval). Although no longer in production, another tibial nerve stimulator, the Urgent-SQ, may occasionally be encountered. Both devices include a passive receiver element with electrodes implanted near the tibial nerve and require an additional external pulse generator that sends power and stimuli to the receiver transcutaneously.
References
Coolen RL, Groen J, Blok BFM. Electrical stimulation in the treatment of bladder dysfunction: technology update. Medical Devices: Evidence and Research 2019:12 337–345.
De Wachter S, Knowles CH, Elterman DS, et al. New technologies and applications in sacral neuromodulation: an update. Adv Ther 2020; 37:637-643. [DOI LINK]
Goldman HB, Lloyd JC, Noblett KL, et al. International Continence Society best practice statement for use of sacral neuromodulation. Neuro Urodynamics 2018;1-26 [DOI LINK]
Janssen DAW, Martens FMJ, de Wall LL, et al. Clinical utility of neurostimulation devices in the treatment of overactive bladder: current perspectives. Medical Devices: Evidence and Research 2017; 10:109-122. [DOI LINK] (provides VOCARE x-ray under CC-BY)
Vignes JR, Bauchet L, Ohanna F. Dorsal rhizotomy combined with anterior sacral root stimulation for neurogenic bladder. Act Neurochir Suppl 2007; 97:323-331. (Description of VOCARE system)
Coolen RL, Groen J, Blok BFM. Electrical stimulation in the treatment of bladder dysfunction: technology update. Medical Devices: Evidence and Research 2019:12 337–345.
De Wachter S, Knowles CH, Elterman DS, et al. New technologies and applications in sacral neuromodulation: an update. Adv Ther 2020; 37:637-643. [DOI LINK]
Goldman HB, Lloyd JC, Noblett KL, et al. International Continence Society best practice statement for use of sacral neuromodulation. Neuro Urodynamics 2018;1-26 [DOI LINK]
Janssen DAW, Martens FMJ, de Wall LL, et al. Clinical utility of neurostimulation devices in the treatment of overactive bladder: current perspectives. Medical Devices: Evidence and Research 2017; 10:109-122. [DOI LINK] (provides VOCARE x-ray under CC-BY)
Vignes JR, Bauchet L, Ohanna F. Dorsal rhizotomy combined with anterior sacral root stimulation for neurogenic bladder. Act Neurochir Suppl 2007; 97:323-331. (Description of VOCARE system)
Related Questions
Can vagal nerve stimulators be safely scanned?
Can vagal nerve stimulators be safely scanned?