Yes. Several immediately come to mind.
|
Cosmetics
All eye make-up, especially mascara, should be removed prior to imaging. Such cosmetics often contain iron oxides that create a local artifact and may even result in eye irritation during MR imaging.
Permanent eye-liner and periorbital tattoos may cause local swelling and pain even hours after scanning. Although some experts have recommended placing cold packs on the eyes preemptively, we simply warn the patient about this possibility and tell them to notify us immediately if any discomfort is experienced.
|
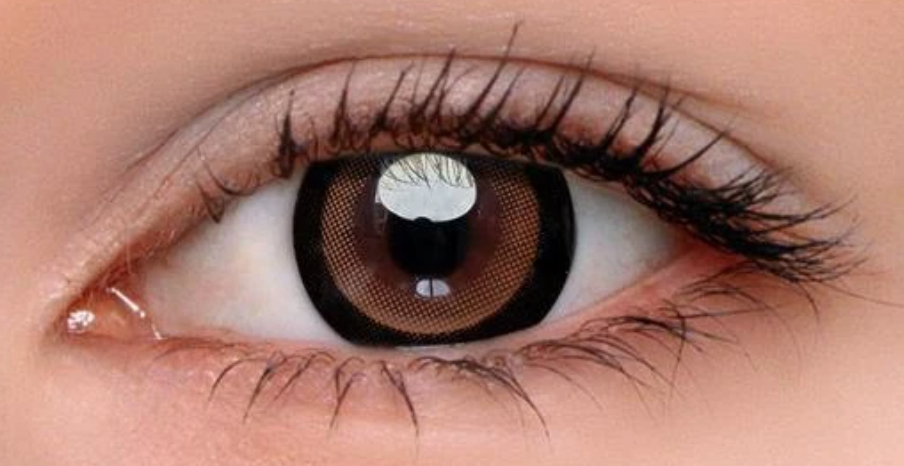
Recently magnetic artificial eyelashes have become available which constitute a potential MR safety hazard as well as a source of artifacts. Instead of glue, the user paints on a black eyeliner containing ultrafine magnetic particles. The lashes themselves contain thin magnetic strips at their bases to attach to the lids. Both the lashes and eyeliner should be removed prior to imaging.
Artificial Lenses
Virtually all intraocular lens (IOL) implants are made entirely of plastic or silicone, and thus present no safety concerns in the MR environment. In a few older models, metallic loops made of platinium, iridium or titanium may be attached to the lens for suturing purposes; however, all tested to date have demonstrated no significant heating or displacement at field strengths up to 7.0T. One relatively new complex IOL, the implantable miniature telescope, is considered MR Conditional because it contains a small stainless steel part in its center, so there are restrictions on maximum field and gradient strength.
|
Conventional external contact lenses are also entirely safe, even the new fully implantable ones. It should be noted that certain cosmetic "circle" lenses contain iron oxide pigments that can produce a localized artifact on MR images (but without apparent adverse health effects).
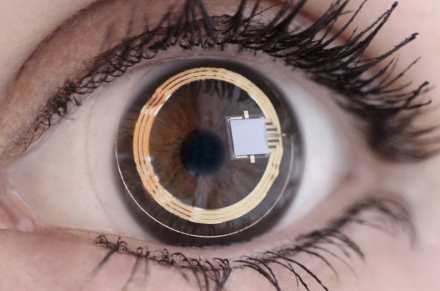
The single MR Unsafe implant in this category is the relatively new Sensimed Triggerfish, a contact lens with an embedded micro-sensor that captures spontaneous circumferential changes at the corneoscleral area (an indirect index for anterior chamber size and hence intraocular pressure). These uncommonly used devices are implanted for a 24-hour period to continuously monitor intraocular pressure in glaucoma patients, then removed.
|
Eyelid Weights and Springs
Internal palpebral weights made of gold, platinum, or platinum-iridium and are inserted in the pretarsal space of the upper lid to passively assist with lid closure in patients with facial nerve palsy. Although most are probably safe even up to 7.0T, some are labeled MR Conditional by manufacturers (out of an abundance of caution, I suppose). Externally applied weights are also available, such as the Blinkeze™ plate held in place by an adhesive strip. Even though made of (non-ferromagnetic) tantalum, such external weights should be removed prior to MRI to eliminate the possibility of skin burns.
|
Implanted palpebral springs, more widely used in the 1970s and 1980s than today, assist with active closure of the lid. These devices are not commercially produced, but each is individually crafted by the surgeon using stainless steel, Nitinol (nickel-titanium), or nonferrous 35 NLT alloy wire. Although some concerns about movement within 1 month of placement and localized discomfort have been voiced, many patients have undergone uneventful MRI at 3T or less with the only precaution being placement of a protective plastic covering and eye patch.
|
Glaucoma Devices
 Metal EX-PRESS glaucoma shunt
Metal EX-PRESS glaucoma shunt
Implanted glaucoma devices are used to reduce intraocular pressure by removing aqueous humor from the anterior chamber of the eye. They are virtually all constructed out of silicone or plastic and are considered MR Safe (with two exceptions):
The first exception is the metal EX-PRESS Glaucoma Filtration Device, a 2-3 mm long pointed stainless steel tube that is implanted under a scleral flap. The tube drains fluid from the anterior chamber into the intrascleral space. The EX-PRESS mimics a foreign body on x-ray and creates a local artifact on MR. It is considered MR Conditional up to 3.0T with the additional caveat that the manufacturer does not recommend scanning in the first 2 weeks post implantation.
The first exception is the metal EX-PRESS Glaucoma Filtration Device, a 2-3 mm long pointed stainless steel tube that is implanted under a scleral flap. The tube drains fluid from the anterior chamber into the intrascleral space. The EX-PRESS mimics a foreign body on x-ray and creates a local artifact on MR. It is considered MR Conditional up to 3.0T with the additional caveat that the manufacturer does not recommend scanning in the first 2 weeks post implantation.
 "MR conditional" Hydrus® microstent
"MR conditional" Hydrus® microstent
The second exception is the Hydrus® Microstent (Ivantis), a crescent-shaped device made of non-ferromagnetic nitinol (an alloy of nickel and titanium) placed in Schlemm’s canal near the corneal-iris junction. (Schlemm’s canal is a lymphatic-like vessel in the deep cornea whose function is to drain aqueous humor from the anterior chamber into the episcleral blood vessels). Like the EX-PRESS Device, the Hydrus is MR Conditional up to 3.0T.
|
Scleral Banding
Scleral buckling or banding is used to treat chronic retinal detachment. The procedure involves encircling the globe with a belt, band, buckle, or tire-like structure made of silicone or hydrogel, occasionally held in place by metallic clip. For the last 30 years, these clips have been made of tantalum, and hence pose no risk of movement in an external magnetic field.
|
Eye Prostheses
|
The typical prosthetic eye consists of: 1) a posterior globe component, commonly made of hydroxyapatite, porous polyethlyene, acrylic, silicone, or bioceramic; and 2) a removable anterior shell, made of acrylic or occasionally glass and painted to resemble the normal eye. The extraocular muscles may or may not be sutured to the implant. Some patients have only a scleral shell without a posterior globe prosthesis. Because none of these components in standard eye prostheses contain metal, they are all considered MR Safe. Some eye prostheses have a titanium peg that couples the anterior and posterior components so they move together. These are also "MR safe".
|
As a warning, a small number of legacy MEDPOR prostheses implanted in the 2000-2010 time frame that contain a small magnet in the shell magnetically coupled to a ferromagnetic screw on the globe component. MR scanning of these prostheses is contraindicated.
Miscellaneous and Uncommon Devices
|
Corneal Implants. The Boston Kerato-prosthesis is designed as an artificial cornea for patients with previous failed graft attempts. The collar-button-like device attaches to the native cornea and contains consists of a clear sheet of plastic holding a donor graft and affixed to a back plate with locking titanium ring. Because of the possible tissue heating near the metal component, the device is considered MR Conditional.
|
|
'Electronic Retinal Implants. Two devices, the Argus II Retinal Prosthesis System and the IRIS II have been granted CE mark for use in Europe (the Argus also has FDA approval in the US). Both devices comprise an epiretinal electrode array located on the surface of the retina together with an eyeglass-mounted digital camera transmitting signals wirelessly to the impant's receiver. With the external hardware removed, both devices are considered MR Conditional at fields up to 3.0T.
|
|
Retinal tacks made of plastic or metal were reasonably popular in the 1980s as an adjunct for repair of complicated retinal detachments. At least one of these tacks, the Western European model, was made of ferromagnetic steel and has long been singled out as MR Unsafe. Retinal tacks are seldom used today because of their general commercial unavailability and associated complications. However, the Argus II Retinal Prosthesis uses a single titanium retinal tack to hold the electrode array in place and is MR Conditional.
|
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Albert DW, Olsen KR Parel J-M, et al. Magnetic resonance imaging and retinal tacks (letter). Arch Ophthalmol 1990; 108:320-321.
Bakshandeh H, Shellock FG, Schatz CJ, Morisoli SM. Metallic clips used for scleral buckling: ex vivo evaluation of ferromagnetism at 1.5T (letter). J Magn Reson Imaging 1993: 3:559. (they are safe)
Luo YH-L, da Cruz L. A review and update on the current status of retinal prostheses (bionic eye). Br Med Bull 2014; 109:31-44. [DOI Link]
Mabray MC, Uzelac A, Talbott JF, et al. Ex-PRESS glaucoma filter: an MRI compatible metallic orbital foreign body imaged at 1.5 and 3 T. Clin Radiol 2015; 70:e28-e34. [DOI LINK]
Reiter MJ, Schwope RB, Kini JA, et al. Postoperative imaging of the orbital contents. RadioGraphics 2015; 35:221-234. [DOI LINK]
Schrom T, Thelen A, Asbach P, Bauknecht H-C. Effect of 7.0 Tesla MRI on upper eyelid implants. Ophthalm Plast Recon Surg 2006; 22:480-482. [DOI Link] (no heating or displacement for three types of eyelid weights tested at 7.0T)
Schwering MS, Oltrup T, Rückheim KS, et al. New retinal tack designs: an analysis of retention forces in human scleral tissue. Graefe's Arch Clin Exp Ophthalmol 2020; 258:1389-1394. [DOI Link]
Seiff SR, Vestel KP, Truwit CL. Eyelid palpebral springs in patients undergoing magnetic resonance imaging: an area of possible concern (letter). Arch Ophthalmol 1991; 109:319.
Slonimsky E, Mamourian A. Magnetic eyelashes: a new source of MRI artifacts. AJR Am J Roentgenol 2019; 213:983-985.
van Rijn GA, Mourik JEM, Teeuwisse WM, et al. Magnetic resonance compatibility of intraocular lenses measured at 7 Tesla. IOVS Invest Ophthalmol Visual Sci 2012; 53:3449-53. [DOI LINK]
Weiland JD, Faraji B, Greenberg Rj, et al. Assessment of MRI issues for the Argus II Retinal Prosthesis. Magn Reson Imaging 2012; 30:382-389.
Yuh WTC, Hanigan MT, Nerad JA, et al. Extrusion of eye socket magnetic implant after MR imaging: potential hazard to patient with eye prosthesis. J Magn Reson Imaging 1991; 1:711-2. (included for historical interest only; the Troutman extruded magnetic implant was used mostly between 1940 and 1960, so there are probably no patients remaining alive with this device).
Albert DW, Olsen KR Parel J-M, et al. Magnetic resonance imaging and retinal tacks (letter). Arch Ophthalmol 1990; 108:320-321.
Bakshandeh H, Shellock FG, Schatz CJ, Morisoli SM. Metallic clips used for scleral buckling: ex vivo evaluation of ferromagnetism at 1.5T (letter). J Magn Reson Imaging 1993: 3:559. (they are safe)
Luo YH-L, da Cruz L. A review and update on the current status of retinal prostheses (bionic eye). Br Med Bull 2014; 109:31-44. [DOI Link]
Mabray MC, Uzelac A, Talbott JF, et al. Ex-PRESS glaucoma filter: an MRI compatible metallic orbital foreign body imaged at 1.5 and 3 T. Clin Radiol 2015; 70:e28-e34. [DOI LINK]
Reiter MJ, Schwope RB, Kini JA, et al. Postoperative imaging of the orbital contents. RadioGraphics 2015; 35:221-234. [DOI LINK]
Schrom T, Thelen A, Asbach P, Bauknecht H-C. Effect of 7.0 Tesla MRI on upper eyelid implants. Ophthalm Plast Recon Surg 2006; 22:480-482. [DOI Link] (no heating or displacement for three types of eyelid weights tested at 7.0T)
Schwering MS, Oltrup T, Rückheim KS, et al. New retinal tack designs: an analysis of retention forces in human scleral tissue. Graefe's Arch Clin Exp Ophthalmol 2020; 258:1389-1394. [DOI Link]
Seiff SR, Vestel KP, Truwit CL. Eyelid palpebral springs in patients undergoing magnetic resonance imaging: an area of possible concern (letter). Arch Ophthalmol 1991; 109:319.
Slonimsky E, Mamourian A. Magnetic eyelashes: a new source of MRI artifacts. AJR Am J Roentgenol 2019; 213:983-985.
van Rijn GA, Mourik JEM, Teeuwisse WM, et al. Magnetic resonance compatibility of intraocular lenses measured at 7 Tesla. IOVS Invest Ophthalmol Visual Sci 2012; 53:3449-53. [DOI LINK]
Weiland JD, Faraji B, Greenberg Rj, et al. Assessment of MRI issues for the Argus II Retinal Prosthesis. Magn Reson Imaging 2012; 30:382-389.
Yuh WTC, Hanigan MT, Nerad JA, et al. Extrusion of eye socket magnetic implant after MR imaging: potential hazard to patient with eye prosthesis. J Magn Reson Imaging 1991; 1:711-2. (included for historical interest only; the Troutman extruded magnetic implant was used mostly between 1940 and 1960, so there are probably no patients remaining alive with this device).
Related Questions
I've heard people have been blinded by metallic foreign bodies in the eye. How do you screen for these?
How do you screen patients for implants and foreign bodies prior to MRI?
I've heard people have been blinded by metallic foreign bodies in the eye. How do you screen for these?
How do you screen patients for implants and foreign bodies prior to MRI?