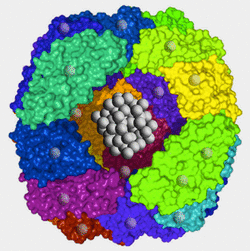
Ferritin is the principal iron storage molecule found in animal cells. It is a globular protein complex about 10 nm in diameter composed of 24 subunits arranged as a hollow shell (apoferritin) in which iron atoms can be packed. A typical ferritin molecule contains about 2000 iron atoms at its core, but potentially may hold up to 4500. Pores are present on the surface of the complex allowing iron atoms to enter and be released from the core. In this manner ferritin is able to regulate levels of intracellular iron.
Each iron atom in ferritin has 3 unpaired electrons. When placed in an external magnetic field, spins from these thousands of electrons lock together through a quantum process known as exchange coupling. The ferritin core acts as a solid piece of metal with a single magnetic domain, a phenomenon called superparamagnetism.
|
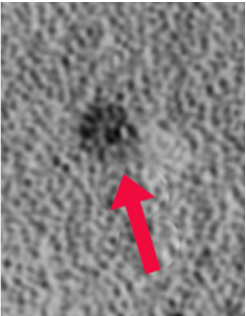
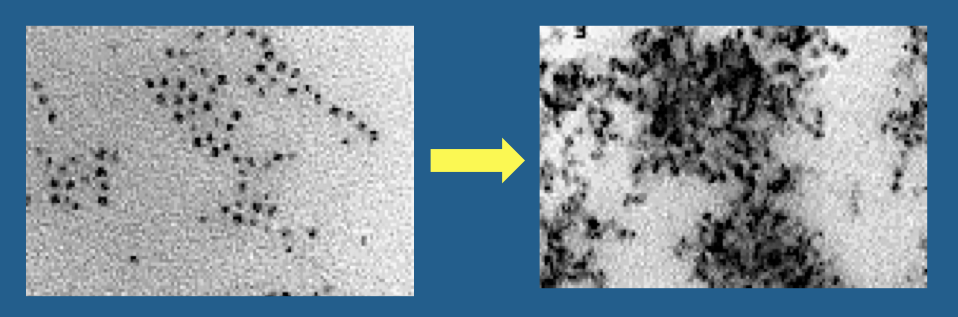
Hemosiderin, in contrast to ferritin, is an amorphous iron-containing substance with no fixed composition. It consists of conglomerates of clumped ferritin particles, denatured proteins, and lipids. The iron within hemosiderin is insoluble, but is in equilibrium with the soluble ferritin pool. Hemosiderin accumulates in macrophages, glial cells, and in those of the reticuloendothelial system. Aggregates reach large size (1-2 μm) and visible under light microscopy
|
Hemosiderin, like ferritin, is superparamagnetic. Because its particles are larger, hemosiderin's magnetic susceptibility effects are even more powerful. Both ferritin and hemosiderin give rise to marked T2/T2* shortening, making areas where they accumulate appear dark on MR images. A more complete discussion of their imaging properties is found in the next Q&A.
Advanced Discussion (show/hide)»
How does iron get in an out of the ferritin shell?
Apoferritin possesses ferroxidase activity that oxidizes soluable ferrous (Fe+2) to insoluable ferric (Fe+3) iron, the form bound by ferritin. Within the shell, iron ions form a crystalline structure with phosphate and hydroxide ions [FeO(OH)]8[FeO(H2PO4)] similar to the mineral known as ferrihydrite. When iron is released from ferritin it is reduced back to the ferrous state prior to transport into the blood.References
Hahn PF, Granick S, Bale WF, Michaelis L. Ferritin: VI. Conversion of inorganic and hemoglobin iron into ferritin iron in the animal body. Storage function of ferritin iron as shown by radioactive and magnetic measurements. J Biol Chem 1943; 150:407-412.
Lawson DM, Artymiuk PJ, Yewdall SJ, et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 1991; 349:541-544.
Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cerebral Blood Flow Metabolism 2003; 23:629-652.
Hahn PF, Granick S, Bale WF, Michaelis L. Ferritin: VI. Conversion of inorganic and hemoglobin iron into ferritin iron in the animal body. Storage function of ferritin iron as shown by radioactive and magnetic measurements. J Biol Chem 1943; 150:407-412.
Lawson DM, Artymiuk PJ, Yewdall SJ, et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 1991; 349:541-544.
Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cerebral Blood Flow Metabolism 2003; 23:629-652.
Related Questions
What's so "super" about superparamagnetism?
What are the different forms of hemoglobin and why do they have different magnetic properties?
What's so "super" about superparamagnetism?
What are the different forms of hemoglobin and why do they have different magnetic properties?