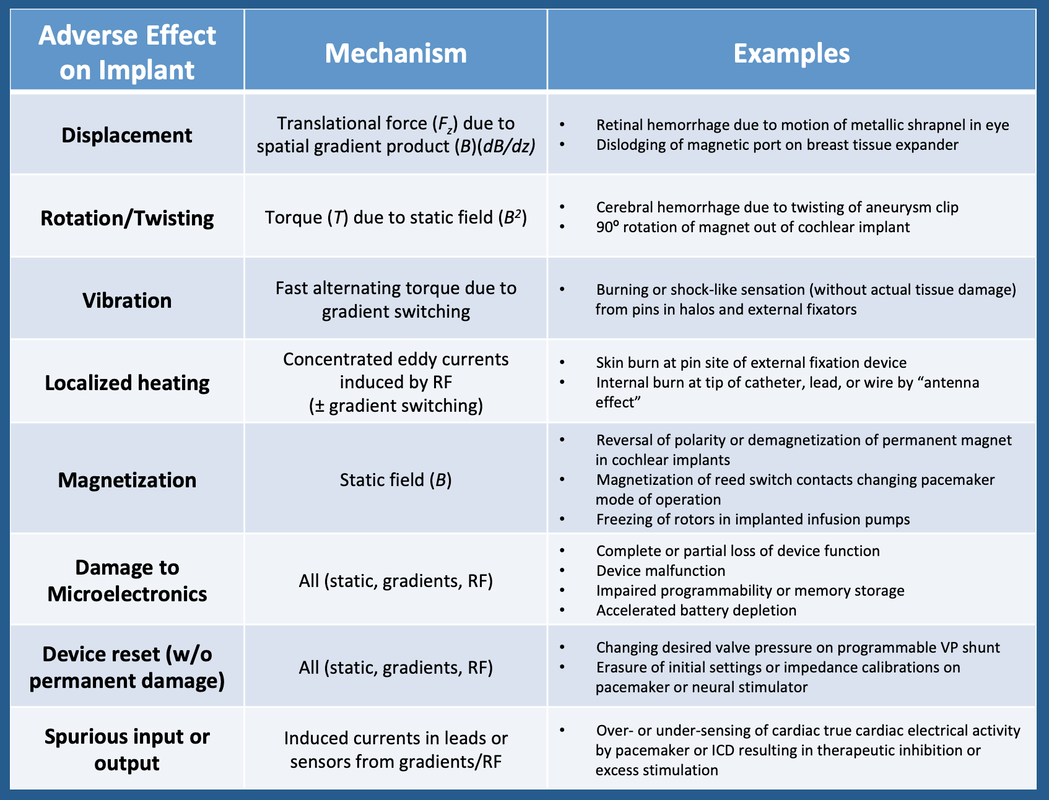
An implant can be considered any foreign material or device located within or attached to the body of a patient. Active implants, also commonly referred to as Active Implantable Medical Devices (AIMDs), are defined to be those requiring external power to operate (excluding energy generated by gravity or the human body itself). Commonly encountered active implants include cardiac pacemakers, internal defibrillators, neurostimulators, and implanted infusion pumps. Passive implants do not require an external power source. Examples include aneurysm clips, stents, hip prostheses, catheters, electric leads, external fixation devices, and IVC filters.
MRI may affect implants through a wide range of mechanisms as categorized in the table below. All risks associated with passive implants (displacement, rotation, heating, and magnetization) apply to active implants as well, but active implants have additional risks. Many of these concepts will be further developed in later Q&A's.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Al-Dayeh L, Rahman M, Venook R. Practical aspects of MR imaging safety test methods for MR conditional active implantable medical devices. Magn Reson Imaging Clinic N Am 2020: 28:559-571. [DOI Link]
Fiek M, Remp T, Reighmann C, Steinbeck G. Complete loss of ICD programmability after magnetic resonance imaging. PACE 2004; 27:1002-1004. [DOI Link]
Hartwell RC, Shellock FG. Letter to the Editor. MRI of cervical fixation devices: sensation of heating caused by vibration of metallic components. J Magn Reson Imaging 1997; 7:771–772. [DOI Link]
Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Health Rhythm 2017; 14:e97-e153. [DOI Link]
ISO/TS 10974:2018. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. International Standards Organization, Geneva, 2018.
Panych LP, Madore B. The physics of MRI safety. J Magn Reson Imaging 2018; 47:28-43. [DOI link]
Seewöster T, Löbe S, Hilbert S, et al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: best practice and real-world experience. Europace 2019; 21:1220-1228. [DOI Link]
Shellock F. MRISafety.com. (The essential resource providing MR safety information and links for over 5000 implanted devices)
Winter L, Seifert F, Zilberti L, et al. MRI-related heating of implants and devices: a review. J Magn Reson Imaging 2020;(in press) [DOI Link]
Young NM, Rojas C, Deng J, et al. Magnetic resonance imaging of cochlear implant recipients. Otol Neurotol 2016; 37:665-671. [DOI Link]
Al-Dayeh L, Rahman M, Venook R. Practical aspects of MR imaging safety test methods for MR conditional active implantable medical devices. Magn Reson Imaging Clinic N Am 2020: 28:559-571. [DOI Link]
Fiek M, Remp T, Reighmann C, Steinbeck G. Complete loss of ICD programmability after magnetic resonance imaging. PACE 2004; 27:1002-1004. [DOI Link]
Hartwell RC, Shellock FG. Letter to the Editor. MRI of cervical fixation devices: sensation of heating caused by vibration of metallic components. J Magn Reson Imaging 1997; 7:771–772. [DOI Link]
Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Health Rhythm 2017; 14:e97-e153. [DOI Link]
ISO/TS 10974:2018. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. International Standards Organization, Geneva, 2018.
Panych LP, Madore B. The physics of MRI safety. J Magn Reson Imaging 2018; 47:28-43. [DOI link]
Seewöster T, Löbe S, Hilbert S, et al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: best practice and real-world experience. Europace 2019; 21:1220-1228. [DOI Link]
Shellock F. MRISafety.com. (The essential resource providing MR safety information and links for over 5000 implanted devices)
Winter L, Seifert F, Zilberti L, et al. MRI-related heating of implants and devices: a review. J Magn Reson Imaging 2020;(in press) [DOI Link]
Young NM, Rojas C, Deng J, et al. Magnetic resonance imaging of cochlear implant recipients. Otol Neurotol 2016; 37:665-671. [DOI Link]
Related Questions
How do you calculate the magnetic force pulling a piece of metal toward the scanner?
What is SAR?
What does it mean when an implant or device is labeled "conditional"?
What causes implants to heat up during MR imaging?
How do you calculate the magnetic force pulling a piece of metal toward the scanner?
What is SAR?
What does it mean when an implant or device is labeled "conditional"?
What causes implants to heat up during MR imaging?