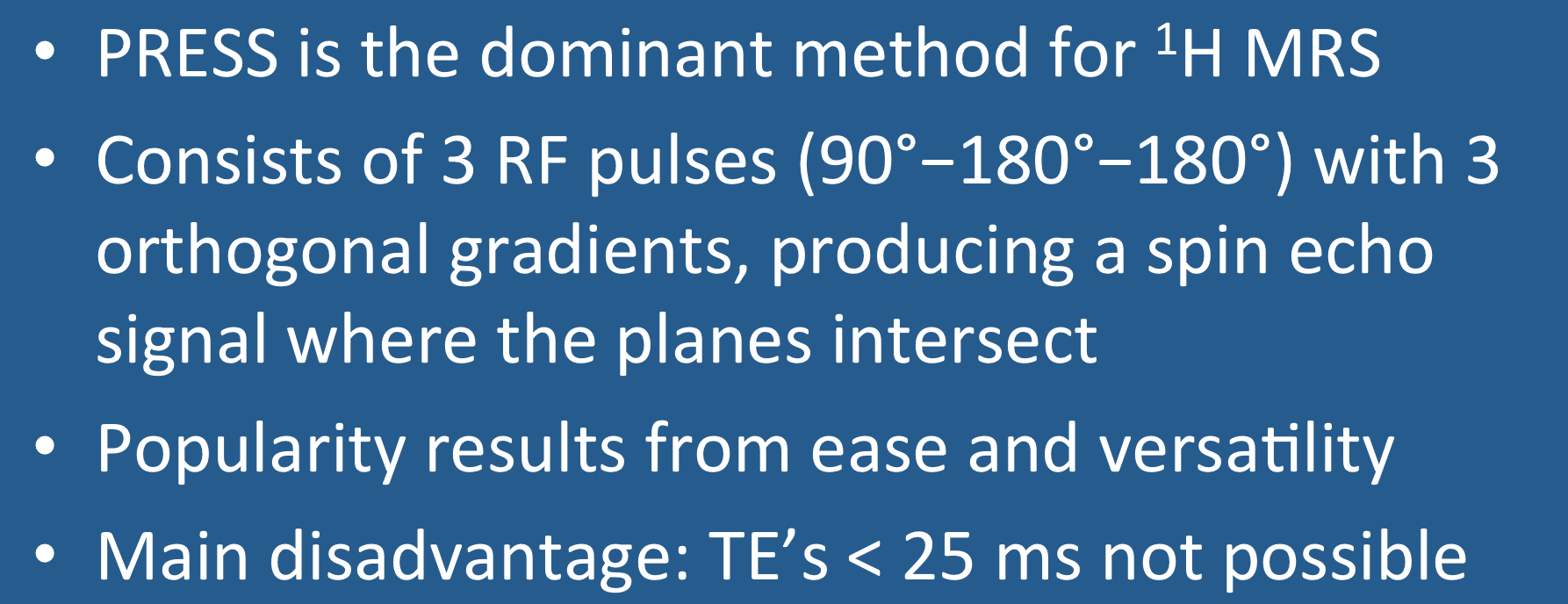
Point RESolved Spectroscopy (PRESS) is the dominant method used for ¹H spectroscopy at 1.5T and 3.0T. The core sequence consists of three slice-selective RF-pulses (90º−180º−180º) applied concurrently with three orthogonal gradients (x, y and z). The PRESS signal at time TE is a spin echo derived only from protons that have experienced all 3 RF-pulses. These protons are located in a cuboid-shaped voxel where the three imaging planes overlap.
The PRESS sequence is relatively easy to program and implement. It is not restricted to single voxel spectroscopy (SVS) but can be used with phase-encoding gradients in chemical shift imaging (CSI) allowing subdivision into multiple smaller voxels.
The main disadvantage of PRESS is limitation of its minimum achievable TE. In practice, TE's of 30-35 msec are commonly used, and values below 25 msec are difficult to attain. The relatively high minimum TE directly follows from the pulse sequence structure -- multiple RF-pulses and waiting for spin-echoes takes time!
The practical implication is that metabolites with short T2's will be difficult to resolve using PRESS. Thus PRESS cannot be used for ³¹P spectroscopy at all (where all the relevant metabolites have very short T2s). And since T2 values decrease with increasing field strength, PRESS is less useful even for ¹H spectroscopy at 7T and above.
A final minor limitation of PRESS is the potential for tissue heating. The multiple 180º-pulses deposit considerable energy, and in some instances specific absorption rate (SAR) limits may be exceeded. In these cases the less commonly used STimulated Echo Acquisition Mode (STEAM) method may offer lower energy deposition (as well as shorter TE's) than PRESS and be preferred.
Advanced Discussion (show/hide)»
A more detailed look at the PRESS pulse sequence
The full PRESS pulse SVS sequence contains some additional features omitted for clarity in the simplified diagram above. Below is a more complete timing diagram as might be implemented on a clinical scanner. Many vendor-specific variations exist especially with regard to crusher gradients, but the diagram below can be considered representative.
At its core, SVS PRESS is simply a 90°−180°−180° double spin-echo pulse sequence. Each RF-pulse is applied simultaneously with a different imaging field gradient (Gz, Gy, or Gx), colored pink, peach, and red above. In our illustratration the first (90°)-pulse is applied concurrently with Gz, exciting protons in a plane perpendicular to the z-axis. The thickness and location of this plane is determined by the strength of Gz together with the center frequency and bandwidth of the RF-pulse. The subsequent 180°-pulses are likewise applied with their own gradients, stimulating protons perpendicular to the y- and x-axes respectively.
Only protons in lying at the intersection of the 3 crossing planes experience all 3 RF-pulses and generate the PRESS echo. The selected planes are usually orthogonal (i.e., mutually perpendicular), so the resultant voxel has a cuboid shape. The sides of this voxel need not be parallel to the scanner's x-, y-, and z-axes, but can be rotated into any plane by applying two or more gradients simultaneously with each RF-pulse. The rotated gradients are nearly always orthogonal to one another but do not have to be; if not, the resultant voxel becomes a parallelepipid rather than a cuboid.
The PRESS echo is sampled without the use of a readout gradient (as would be done in a conventional imaging experiment). This is because frequency differences in the signal must be used to calculate chemical shifts rather than being used for spatial encoding. The PRESS echo is somewhat asymmetric, and often only the second portion is sampled as an FID before being Fourier transformed to produce a spectrum. The leading edge of the PRESS echo is often affected by the crusher gradients used to reduce spurious signals. Additionally, using the echo maximum as the first time domain sample point simplifies analysis of J-coupled metabolites and makes first order phase correction unnecessary during data post-processing. Furthermore, to extract maximum possible frequency information from this signal, a much longer sampling window is used than in MR imaging, sometimes up to several hundred milliseconds.
In addition to the final PRESS echo, two intermediate MR signals are generated (an FID and Echo 1) that are not separately sampled. The FID results from the action of the initial 90°-pulse that tips magnetization into the transverse plane. Echo1 is a spin-echo that results from the combination of the 90°- and first 180°-pulses; it occurs at time TE1, precisely at twice the interval between the first two pulses. Note that Echo 1 arises from the column of voxels along the intersection of the first two planes, since only protons in these voxels have experienced both RF-pulses.
The final PRESS signal is a spin echo generated by the refocusing action of the second 180°-pulse on the subset of Echo 1 spins that also lie in the third crossing plane. The PRESS echo forms at time TE2 after the center of Echo 1, with the last 180°-pulse halfway in between. The total echo time (TE) for the PRESS sequence is therefore TE = TE1 + TE2.
The need for three RF-pulses limits the shortest possible TE for the PRESS sequence. On commercially available clinical MR systems today, the minimum TE for PRESS is in the range of 20-30 msec. This is achieved by setting TE1 = TE2 = ½TE. Conversely, much longer TE's (into the hundreds of msec) are easily attainable by merely extending TE1, TE2, or both.
Crusher gradients (blue) are required to eliminate spurious signals from contaminating the PRESS spectrum. The size, location, and timing of these gradients varies among vendors, but a typical configuration called a "crusher pair" flanking each 180°-pulse is shown. The purpose of crusher gradients is to destroy unintended FIDs and echoes arising outside the volume of interest due to imperfect RF-pulses.
In theory, an ideal 180°-pulse should only produce an in-plane flip of the transverse magnetization. However, 180°-pulses are notoriously imperfect, especially along their edges, so some spins in the imaged volume may be flipped less or more than 180°. This means that some additional longitudinal magnetization may be inadvertently brought down into the transverse plane. This magnetization could produce an FID after the ≈180°-pulse or, even more insidiously, join another coherence pathway to appear later in the sequence as an unwanted stimulated or spin echo.
The right crusher (after the 180°) destroys this unwanted FID and disrupts the phase of undesired pathways. Because this right crusher would also scramble the phases of spins giving rise to the desired PRESS signal, a left crusher is needed before the 180°-pulse to balance its effect. The left crusher does not affect the transverse magnetization but predictably dephases spins in the desirable signal pathway; the right crusher rephases these desirable spins while destroying the unwanted ones.
References
Bottomley PA. Selective volume method for performing localized NMR spectroscopy. US Patent #4,480,228 (approved 30 Oct 1984). (First description of the method later to known as PRESS).
Klose U. Measurement sequences for single voxel proton MR spectroscopy. Eur J Radiol 2008; 67:194-201.
Moonen CT, von Kienlin M, van Zijl PC, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed 1989; 2:201–207.
Bottomley PA. Selective volume method for performing localized NMR spectroscopy. US Patent #4,480,228 (approved 30 Oct 1984). (First description of the method later to known as PRESS).
Klose U. Measurement sequences for single voxel proton MR spectroscopy. Eur J Radiol 2008; 67:194-201.
Moonen CT, von Kienlin M, van Zijl PC, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed 1989; 2:201–207.
Related Questions
If frequency-encoding cannot be used to determine spatial position, how do you localize an MRS signal?
How does the STEAM method for MR Spectroscopy work and when should it be used?
How do you choose between a single and multi-voxel technique?
If frequency-encoding cannot be used to determine spatial position, how do you localize an MRS signal?
How does the STEAM method for MR Spectroscopy work and when should it be used?
How do you choose between a single and multi-voxel technique?