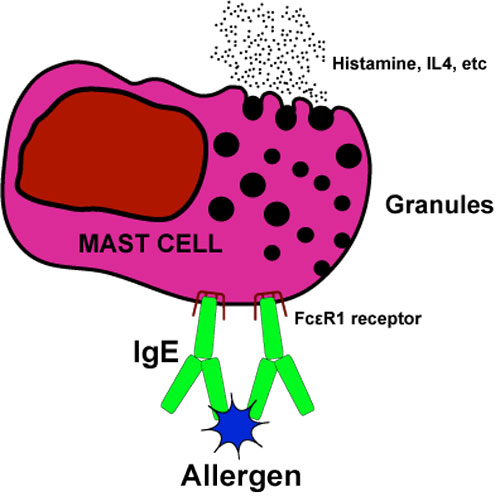
The term anaphylaxis refers to an acute allergic reaction to a drug, food, or other foreign material (the allergen). Clinical characteristics include involvement of the skin and mucosal tissues with hives or swelling, respiratory distress (dyspnea, bronchospasm), and/or circulatory disturbances (reduced blood pressure, syncope, cardiovascular collapse).
|
"True" anaphylaxis is an IgE-mediated type 1 hypersensitivity reaction. Upon first exposure to the allergen (or a mimic antigen with similar molecular surface characteristics), lymphocytes are primed to to produce a specific IgE, but a systemic anaphylactic reaction does not occur. Upon second exposure, however, an amplified response occurs as IgE produced from the first round exposure attaches to the allergen as well as to specific (FcεR1) surface receptors) on mast cells and basophils. This triggers calcium influx with degranulation and release of vasoactive amines (histamines, serotonin, tryptase) and inflammatory mediators (prostaglandins, cytokines, IL4, and leukocyte chemotactic factors).
|
Anaphylactoid reactions have an identical clinical pattern to "true" anaphylaxis but are not IgE-mediated. Here allergens trigger the mast cells to release their granular contents into the circulation but without prior exposure or production of IgE. The allergen may activate mast cells directly or through a number of endogenous (non-IgE) intermediaries, including complement fragments C3a, C4a, C5a (also known as anaphylotoxins); substance P; angiotensin II; endorphins; eosinophilic proteins MBP and ECP; interleukins IL-1 and IL-3; and TNF. Of these, the complement-related pathways seem to be the most important for most pharmaceuticals including contrast agents.
Because true anaphylaxis and anaphylactoid reactions have the same clinical manifestations, sophisticated immunological testing is required (but seldom performed) to determine the exact nature of an allergic reaction to gadolinium. First exposure allergic reactions to gadolinium would seem statistically more likely to be anaphylactoid, but some first exposure IgE-mediated cases have been reported.
Perhaps the only relevant feature of potential clincial importance is that anaphylactoid reactions are dose-related, whereas "true" anaphylaxis reactions are not. If a patient has had a previous mild reaction to a gadolinium contrast agent, in addition to premedication it is worthwhile considering reducing the dose and changing to a different agent. The lower dose strategy might reduce the risk of an anaphylactoid reaction (though it would have no effect on true anaphylaxis). Changing agents could conceivably reduce the risk of both types of reaction.
As a final note, the term "anaphylactoid" is no longer widely used in current allergy/immunology literature, being replaced by the phrase "non-IgE-mediated anaphylaxis". I suspect the radiology community will eventually come around to this more modern nomenclature, but for now anaphylactoid remains ingrained in our literature.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Baxter AB, Lazarus SC, Brasch RC. In vitro histamine release induced by magnetic resonance imaging and iodinated contrast media. Invest Radiol 1993; 28:308-12. (shows concentration for direct histamine release is on order of 100mM, many times the IV concentration of administered Gd. However, complement-mediated pathways were not considered in this experiment.)
Hasdenteufel F, Luyasu S, Renaudin J-M, et al. Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J Allergy Clin Immunol 2008; 121:527-8.
Schiavino D, Murzilli F, Del Ninno M, et al. Demonstration of an IgE-mediated immunological pathogenesis of a severe adverse reaction to gadopentetate dimeglumine. J Invest Allergol Clin Immunol 2003; 13:140-2. (IgE mechanism demonstrated by Prausnitz-Küstner passive transfer test)
Simons FER. Anaphylaxis. J Allergy Clin Immunol 2010; 125:S161-S181.
Thomsen HS. How to manage (treat) immediate-type adverse reactions to GBCA. Top Magn Reson Imaging 2016; 25:269-274. [DOI Link] (Practical review)
Baxter AB, Lazarus SC, Brasch RC. In vitro histamine release induced by magnetic resonance imaging and iodinated contrast media. Invest Radiol 1993; 28:308-12. (shows concentration for direct histamine release is on order of 100mM, many times the IV concentration of administered Gd. However, complement-mediated pathways were not considered in this experiment.)
Hasdenteufel F, Luyasu S, Renaudin J-M, et al. Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J Allergy Clin Immunol 2008; 121:527-8.
Schiavino D, Murzilli F, Del Ninno M, et al. Demonstration of an IgE-mediated immunological pathogenesis of a severe adverse reaction to gadopentetate dimeglumine. J Invest Allergol Clin Immunol 2003; 13:140-2. (IgE mechanism demonstrated by Prausnitz-Küstner passive transfer test)
Simons FER. Anaphylaxis. J Allergy Clin Immunol 2010; 125:S161-S181.
Thomsen HS. How to manage (treat) immediate-type adverse reactions to GBCA. Top Magn Reson Imaging 2016; 25:269-274. [DOI Link] (Practical review)
Related Questions
How safe are gadolinium contrast agents?
How safe are gadolinium contrast agents?