|
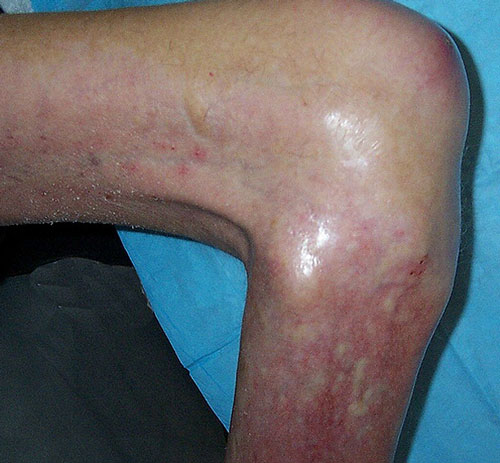
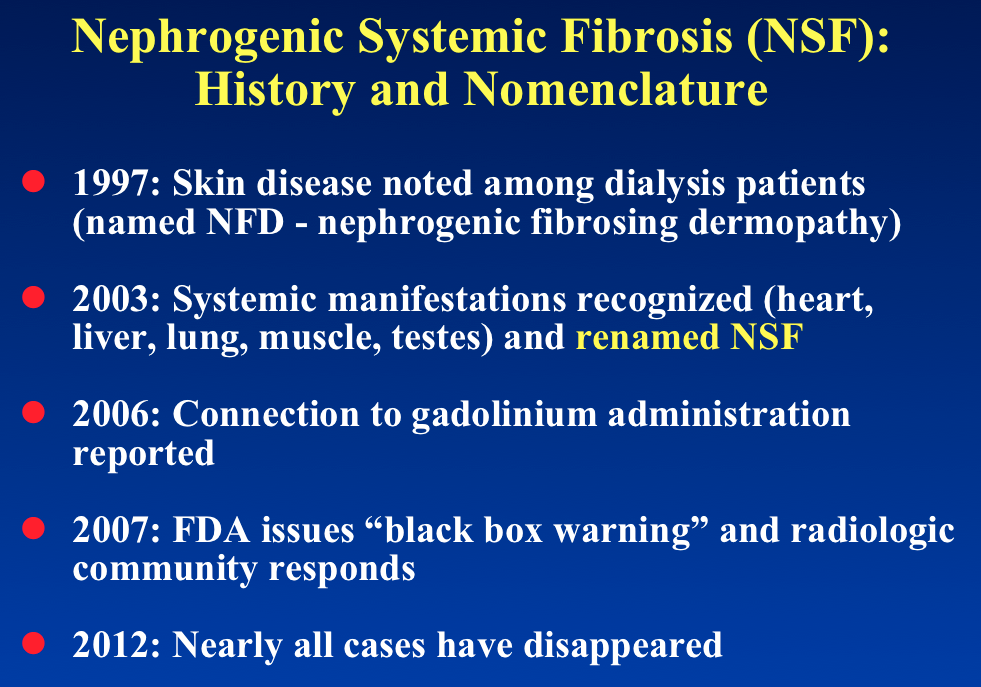
Nephrogenic systemic fibrosis (NSF) is a rare, progressive, often fatal disease characterized by skin thickening, painful joint contractures, and fibrosis of multiple organs including the lungs, liver, muscles, and heart. Nearly all documented cases have occurred in patients with chronic severe renal insufficiency who have received gadolinium contrast. The association between gadolinium and NSF was first reported by Danish nephrologists in 2006. Between 2006 and 2010 several hundred cases were diagnosed worldwide.
|
|
NSF usually develops clinically within days to months following gadolinium exposure, although rare cases have been reported years later. Nearly all patients have been in various degrees of renal failure and many were on dialysis. Only exceedingly rare cases have been reported in patients with eGFRs > 30 mL/min/1.73m². High and/or multiple doses of contrast are frequently reported, as are the use of linear contrast agents (ACR Group I). Other risk factors include liver diease and acidosis.
|
The strong association with renal insufficiency most likely relates to the prolonged biological half-life due to prolonged excretion of gadolinium. However, other factors have been imputed, including metabolic acidosis; elevated iron and phosphate levels; erythropoietin therapy; vasculopathy; and infectious/inflammatory mediators.
The pathogenesis of NSF is believed to begin with the displacement of the Gd ion from its chelate by another metallic cation (Fe+3, Zn+2, or Ca+2) through a so-called transmetalation reaction :
(Metal ion) + Gd-Chelate ↔ Metal-Chelate + Gd+3
The free Gd ion is then deposited in the skin and other soft tissues. There it is engulfed engulfed by CD163+ iron-recycling and other macrophages creating an inflammatory response and cytokine release. Circulating fibrocytes (immunologically unique CD-34 positive cells derived from bone marrow) deposit in tissue, transforming into spindle cells that proliferate and become the hallmark of the disease.
Following recognition of this disorder and its association with gadolinium in patients with renal insufficiency, the worldwide radiology community responded immediately to put an end to this iatrogenic disease. Today, NSF has been nearly completely eliminated due to these measures. In more recent times, however, gadolinium-induced plaques have been reported in the extremities not meeting the full criteria for NSF.
The story of NSF is sad one that we radiologists created. It should serve as a lesson that even drugs which appear to be extraordinarily safe may not be infinitely safe for all patients. Sometimes adverse effects may be subtle, disguised, or appear at long time intervals following administration. NSF is thus a call and reminder to be forever vigilant.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Attari H, Cao Y, Elmholdt TR, et al. A systematic review of 639 patients with biopsy-confirmed nephrogenic system fibrosis. Radiology 2019; 292:376-386.
Abraham JL, Thakral C, Skov L et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetalation and long term persistence in nephrogenic systemic fibrosis. Br J Dermatol 2008; 158: 273–280.
Bennett CL, Qureshi ZP, Sartor AO, Norris LB, Murday A, Xirasagar S, Thomsen HS. Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J. 2012; 5: 82–88.
Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol 2008; 66:191-199.
Elster AD. Energy-dispersive x-ray microscopy to trace gadolinium in tissues. Radiology 1989; 173:868-870. (Method I developed 25 years ago now used to demonstrate Gd accumulation in tissues).
Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques. A new, distinctive clinical entity. JAMA Dermatology 2015; 151:316–319. (rarely, isolated plaques may occur on the extremities, not meeting full criteria for NSF).
Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21: 1104–1108.
Krefting I, FDA. Gadolinium-based contrast agents (GBCAs) and the NSF risk: update. 21 January 2011. US Food and Drug Administration. www.fda.gov
Martin DR. Nephrogenic system fibrosis: a radiologist's practical perspective. Eur J Radiol 2008; 66:220-224.
Martino F, Amici G, Rostner M, et al. Gadolinium-based contrast media nephrotoxicity in kidney impairment: the physiopathological conditions for the perfect murder. J Clin Med 2021; 10:271. [DOI LINK] (good recent review article on the subject).
Peak AS, Sheller A. Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis. Ann Pharmacother 2007; 41:1481–5.
Prasad SR, Jagirdar J. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy: a primer for radiologists. J Comput Assist Tomogr 2008; 32:1-3.
Schroeder JA, Weingart C, Coras B, et al. Ultrastructural evidence of dermal gadolinium deposits in a patient with nephrogenic systemic fibrosis and end-stage renal disease. Clin J Am Soc Nephrol 2008; 3:968-975.
Swaminathan S. Gadolinium toxicity: iron and feroportin as central targets. Magn Reson Imaging 2016; 34:1373-1376.
Attari H, Cao Y, Elmholdt TR, et al. A systematic review of 639 patients with biopsy-confirmed nephrogenic system fibrosis. Radiology 2019; 292:376-386.
Abraham JL, Thakral C, Skov L et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetalation and long term persistence in nephrogenic systemic fibrosis. Br J Dermatol 2008; 158: 273–280.
Bennett CL, Qureshi ZP, Sartor AO, Norris LB, Murday A, Xirasagar S, Thomsen HS. Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J. 2012; 5: 82–88.
Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol 2008; 66:191-199.
Elster AD. Energy-dispersive x-ray microscopy to trace gadolinium in tissues. Radiology 1989; 173:868-870. (Method I developed 25 years ago now used to demonstrate Gd accumulation in tissues).
Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques. A new, distinctive clinical entity. JAMA Dermatology 2015; 151:316–319. (rarely, isolated plaques may occur on the extremities, not meeting full criteria for NSF).
Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21: 1104–1108.
Krefting I, FDA. Gadolinium-based contrast agents (GBCAs) and the NSF risk: update. 21 January 2011. US Food and Drug Administration. www.fda.gov
Martin DR. Nephrogenic system fibrosis: a radiologist's practical perspective. Eur J Radiol 2008; 66:220-224.
Martino F, Amici G, Rostner M, et al. Gadolinium-based contrast media nephrotoxicity in kidney impairment: the physiopathological conditions for the perfect murder. J Clin Med 2021; 10:271. [DOI LINK] (good recent review article on the subject).
Peak AS, Sheller A. Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis. Ann Pharmacother 2007; 41:1481–5.
Prasad SR, Jagirdar J. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy: a primer for radiologists. J Comput Assist Tomogr 2008; 32:1-3.
Schroeder JA, Weingart C, Coras B, et al. Ultrastructural evidence of dermal gadolinium deposits in a patient with nephrogenic systemic fibrosis and end-stage renal disease. Clin J Am Soc Nephrol 2008; 3:968-975.
Swaminathan S. Gadolinium toxicity: iron and feroportin as central targets. Magn Reson Imaging 2016; 34:1373-1376.
Related Questions
Is gadolinium contrast nephrotoxic? Can it be given safely to patients with mild renal insufficiency?
Do all MR contrast agents carry the same risk of NSF?
Is gadolinium contrast nephrotoxic? Can it be given safely to patients with mild renal insufficiency?
Do all MR contrast agents carry the same risk of NSF?