Intrauterine Devices (IUDs)
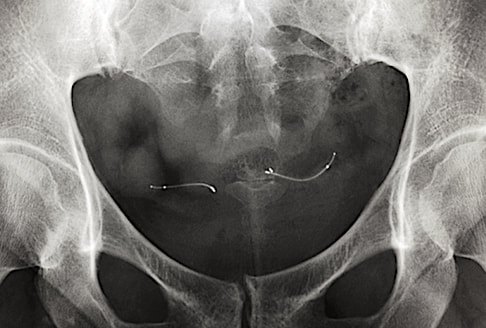
 "MR Conditional" Paragard® Copper T IUD
"MR Conditional" Paragard® Copper T IUD
Currently implanted IUDs are of two types: plastic and metal. All plastic IUDs, such as the commonly used Mirena®, Skyla®, and Liletta®, achieve their contraceptive effects through release of a hormone (levonorgestrel) and are by definition MR Safe. Metal IUDs, such as the commonly prescribed ParaGard®, GoldLuna®, and Gynefix®, rely on copper to impair sperm motility. Some also contain plastic, gold, or Nitinol. A large number of these devices have been formally tested and appear to offer no problems for imaging at least up to 3.0T.
 "MR Unsafe" Chinese steel IUD
"MR Unsafe" Chinese steel IUD
The only IUDs deemed MR Unsafe are stainless-steel ring-shaped devices distributed exclusively in China between 1988 and 2000. Because millions of such IUDs were implanted, a reasonable possibility exists that a middle-aged or older Chinese woman might still have one in place. If reliable medical history is unavailable, these devices should be easily identifiable on x-ray by their opacity and circular shape.
Barrier Devices
Barrier contraceptive devices include sponges, female condoms, and diaphragms. Composed primarily of latex and other plastics, these devices may all be scanned without concern. Many diaphragms contain a flexible metal ring, but to my knowledge no movement, pain, heating or unexpected pregnancies have resulted from their use in the MR environment.
Tubal Ligation/Occlusion Devices
Tubal rings (Falope, Yoon, Lay) are small silastic bands placed around a loop of the fallopian tube. They are MR Safe.
Tubal clips are clamped externally across the fallopian tube to close its lumen. The popular Filshie®
clips are made of titanium, while the Hulka®/Wolf® clips are plastic with a stainless steel spring. Both are MR Conditional up to 3.0T.
clips are made of titanium, while the Hulka®/Wolf® clips are plastic with a stainless steel spring. Both are MR Conditional up to 3.0T.
Essure® devices are metal/fiber coils previously used to internally occlude the fallopian tubes. Although removed from the market in 2018, these are "lifetime" devices for patients having received them. They are MR Conditional at 3.0T and below.
The AltaSeal® (AltaScience), like the Essure®, is a permanent fallopian tube occlusion device placed by hysteroscopy. Used in Europe (but not yet FDA-approved for the US), the AltaSeal® is a 300-series stainless steel micro-insert similar to a coronary stent. It is MR Conditional .
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Buhling KJ, Zite NB, Lotte P, et al. Worldwide use of intrauterine contraception: a review. Contraception 2014; 89:162-173. [DOI LINK]
Bussman S, Luechinger R, Fröhlich JM, et al. Safety of intrauterine devices in MRI. PLoS ONE 2018; 13:30204220. [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials- part 2: neurologic and genitourinary devices and materials. Diagn Interv Radiol 2020; 26:160–167 [DOI LINK]
Neumann W, Uhrig T, Malzacher M, et al. Risk assessment of copper-containing contraceptives: the impact for women with implanted intrauterine devices during clinical MRI and CT examinations. Eur Radiol 2019; 29:2812-2820. [DOI LINK]
Shellock FG. New metallic implant used for permanent contraception in women: evaluation of MR safety. AJR Am J Roentgenol 2002; 178:1513-1516. [DOI LINK] (Safety of ESSURE device up to 1.5T)
Buhling KJ, Zite NB, Lotte P, et al. Worldwide use of intrauterine contraception: a review. Contraception 2014; 89:162-173. [DOI LINK]
Bussman S, Luechinger R, Fröhlich JM, et al. Safety of intrauterine devices in MRI. PLoS ONE 2018; 13:30204220. [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials- part 2: neurologic and genitourinary devices and materials. Diagn Interv Radiol 2020; 26:160–167 [DOI LINK]
Neumann W, Uhrig T, Malzacher M, et al. Risk assessment of copper-containing contraceptives: the impact for women with implanted intrauterine devices during clinical MRI and CT examinations. Eur Radiol 2019; 29:2812-2820. [DOI LINK]
Shellock FG. New metallic implant used for permanent contraception in women: evaluation of MR safety. AJR Am J Roentgenol 2002; 178:1513-1516. [DOI LINK] (Safety of ESSURE device up to 1.5T)
Related Questions
How about other GU devices like nephrostomy tubes and stents?
How about other GU devices like nephrostomy tubes and stents?