|
Diaphragm Stimulation
Several commercially available implanted devices can be used to stimulate the diaphragm, either directly or indirectly through the phrenic nerves. Phrenic nerve stimulators include the Mark IV Breathing Pacemaker (Avery Biomedical), the remedē System (Respircardia), the Atrostim PNS (Atrostim OY, available only in Europe), and the temporary transvenous Lungpacer stimlulator (Lungpacer). The Synapse NeuRx (Synapse Biomedical) and its temporary version, the TranAeris, are unique in that they directly stimulate the diaphragm musculature rather than the phrenic nerve. Regardless of method all current diaphragm stimulators are considered MR Unsafe (except for the remedē System, that is MRI Conditional).
|
Upper Airway Stimulation
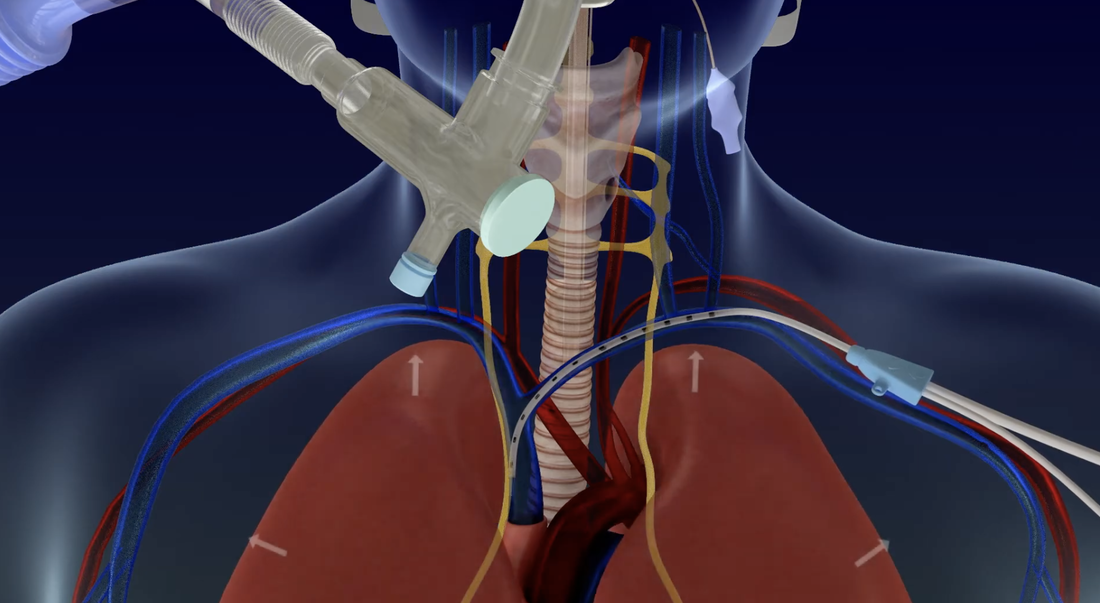
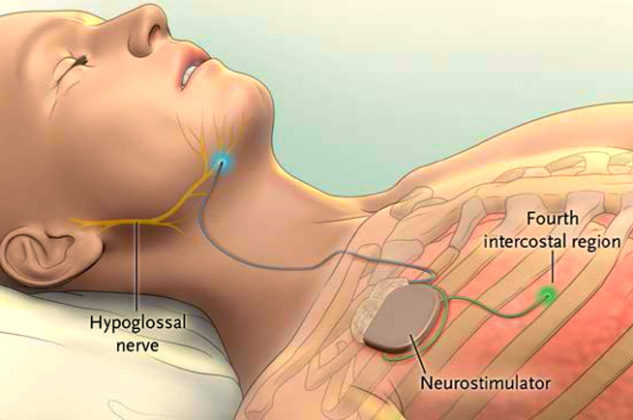
Three upper airway stimulation (UAS) devices are currently available to treat obstructive sleep apnea. Although somewhat different in design, each of these implants electrically stimulates the hypoglossal nerve. This stimulation produces contraction of the genioglossus muscle pulling the tongue forward to open the airway.
|
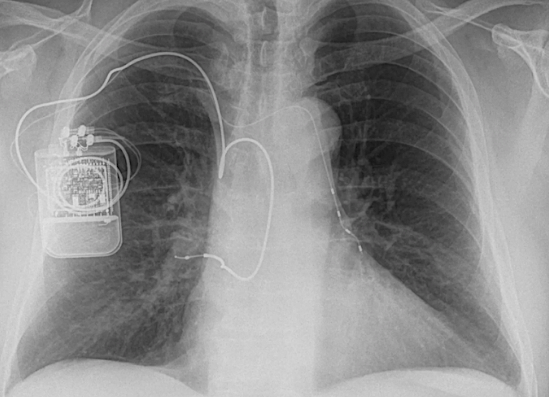
The Inspire® (Inspire Medical) is the most widely used UAS device having both FDA and CE Mark approval. A titanium-encased, battery-powered neurostimulator is subcutaneously implanted in the upper chest. Respiration is monitored by an electrode embedded in the intercostal muscles, with electrical pulses transmitted to stimulate the hypoglossal nerve during apneic episodes. Earlier versions of the implant are MR Unsafe, but the more recent Model 3028 is MR Conditional at 1.5T for head and extremity scanning only.
|
The ImThera aura6000® (LivaNova) has an implanted pacemaker-like device in the upper chest but without a respiratory monitoring lead. The device intermittently sends impulses to six electrodes implanted around the hypoglossal nerve to increase muscular tone of the tongue during sleep. The aura6000® is CE marked but not yet FDA-approved. The manufacturer deems it MR Unsafe.
|
The Genio® system (Nyxoah SA) is the newest upper airway stimulator to receive FDA and CE Mark approval. The device consists of a paddle electrode array touching both hypoglossal nerve branches immediately adjacent to their termination in the genioglossus muscles. Stimulation is achieved transcutaneously by means of an external transmitter device. The Genio® is MR Conditional 6-weeks after implantation at 1.5T and 3.0T, with very specific requirements based on body area imaged. Of course, the external stimulator device must be removed prior to entry into the MR suite.
|
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Eastwood PR, Barnes M, MacKay SG, et al. Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. Eur Resp J 2020; 55:1901320. [DOI LINK]
European Network for Health Technology Assessment (EUnetHTA). Hypoglossal nerve stimulation systems for treatment of obstructive sleep apnea, Version 4.0, 12 June 2020.
Sterman J, Cuáquero A, Dym RJ, et al. Implantable electronic stimulation devices from head to sacrum: imaging features and functions. RadioGraphics 2019; 39:1056-1074. [DOI LINK]
Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139-149. [DOI LINK]
Eastwood PR, Barnes M, MacKay SG, et al. Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. Eur Resp J 2020; 55:1901320. [DOI LINK]
European Network for Health Technology Assessment (EUnetHTA). Hypoglossal nerve stimulation systems for treatment of obstructive sleep apnea, Version 4.0, 12 June 2020.
Sterman J, Cuáquero A, Dym RJ, et al. Implantable electronic stimulation devices from head to sacrum: imaging features and functions. RadioGraphics 2019; 39:1056-1074. [DOI LINK]
Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139-149. [DOI LINK]
Related Questions
Can patients with ET tubes and other upper airway devices undergo MRI?
Can patients with ET tubes and other upper airway devices undergo MRI?