|
Across the entire periodic table, nuclear spin values ranging from I = 0 to I = 8 in ½-unit increments can be found. Protons and neutrons each have net spins of ½, but this derives from the elementary quarks and gluons of which they are composed. As a result of this complexity, no simple formula exists to predict I based on the number of protons and neutrons within an atom. Nevertheless, there are some general rules that apply to special cases:
|
1) Even/Even. Nuclei containing even numbers of both protons and neutrons have I = 0 and therefore cannot undergo NMR. Examples include 4He,12C,16O and 32S.
2) Odd/Odd. Nuclei with odd numbers of both protons and neutrons have spin quantum numbers that are positive integers. Examples include14N (I=1),2H (deuterium, I=1), and10B (I=3).
3) All others. The remaining nuclei (odd/even and even/odd) all have spins that are half integral. Examples include 1H (I=½), 17O (I=5/2), 19F (I=½), 23Na (I=3/2), and 31P (I=½).
For example, the most common naturally occurring isotope of carbon (¹²C) contains 6 protons and 6 neutrons. According to Rule #1 above, ¹²C has I = 0 and cannot undergo NMR. However, carbon's ¹³C isotope (natural abundance 1.07%) contains one extra neutron, resulting in a net spin (I =½) and permitting its use in NMR.
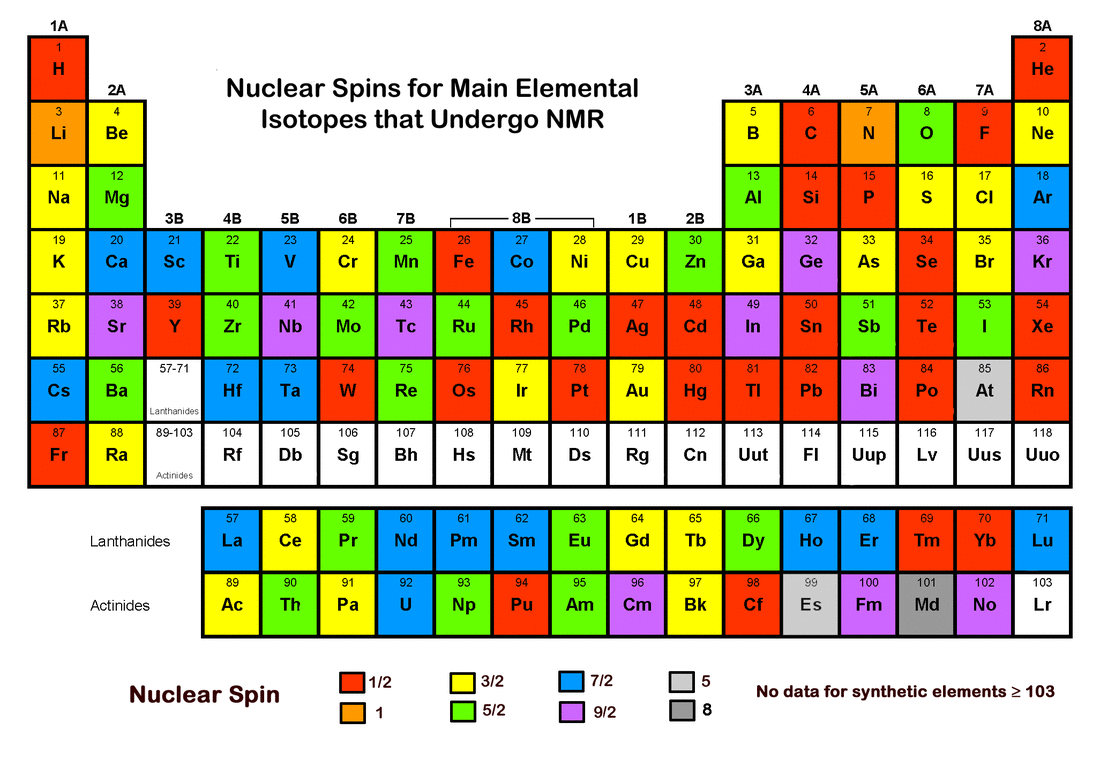
The illustration below shows the distribution of nuclear spin (I) values for principal NMR active isotopes across the periodic table. Some additional "fun facts" about spin are included in the Advanced Discussion section for those interested chemists among you!
Advanced Discussion (show/hide)»
Some "fun facts" about spin. . .
In general, the most abundant isotopes of elements with even atomic numbers all have I = 0, the only exception being beryllium (Z=4). Its most abundant naturally occurring isotope is 9Be, with four protons and five neutrons, giving it a spin (I) = 3/2.
As can be seen from the periodic table above, isotopes with integer values of spin are very uncommon. The only nuclide with I = 2 is 204Tl, an unstable isotope with half-life of 3.5 yrs.
Elements and isotopes with very high values of spin (i.e., I ≥ 7) are relatively rare. Four minor isotopes of technetium, lutetium, and rhenium all have I = 7. They are 94Tc, 96Tc, 176Lu, and 182Re. Three isotopes have the highest possible value for spin (i = 8): niobium (90Nb), tantalum (181Ta), and mendelevium (258Md). The latter (258Md) is the most abundant isotope of mendelevium, which makes Md the "winner" for maximum spin for common isotopes on the periodic table.
Grushow A. NMR Periodic Table (website). Rider University, 2003. (An interesting set of periodic tables that allow you to explore elements sorted by NMR frequency, spin, receptivity, and natural abundance).
Moskowitz C. Proton spin mystery gains a new clue. Scientific American online, Jul 21, 2014. (the important role of gluons in contributing to proton spin)
Parella T. eNMR: NMR Periodic Table (website), 2003. (A fairly complete table with hyperlinks giving NMR properties of approximately 80 elements and their isotopes).
Wood C, Sherman M. Inside the proton, the ‘most complicated thing you could possibly imagine.' Quanta Magazine, October, 2022. [LINK FOR MULTIMEDIA VERSION]
Winter M. WebElements Periodic Table (website), 2013. (A more complete table of all the elements with all their physical properties, including many of the newer, collider-generated ones with atomic numbers > 105. Although NMR data for these elements is lacking, they have non-zero spins and in theory should undergo NMR).