|
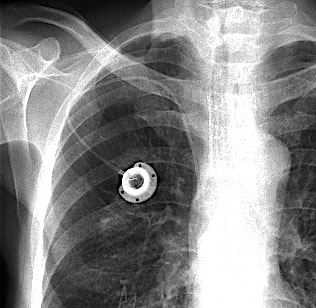
A port is a device for vascular access typically implanted in the upper chest. It consists of two parts: a subcutaneous reservoir made of plastic, titanium, or stainless steel enclosing a self-sealing silicone bubble for needle insertion; and a catheter connected to the reservoir whose tip is placed fluroscopically into the superior vena cava or other major thoracic vein.
At least a dozen companies produce these devices, the largest being AngioDynamics, Braun Medical, Bard, Cook Medical, MedComp, Navilyst Medical, and Smiths Medical. Common brand names include Port-a-Cath, Clip-a-Port, Infuse-a-Port, Medi-Port, and PowerPort.
|
To my knowledge, none of these commercially available portal systems is rated MR Unsafe. Those with metal reservoirs are by definition MR Conditional (with relatively minor restrictions for imaging at 3T or less). Patients with metal-containing ports should be warned that they may feel a pulling or tugging sensation when placed in the scanner. However, because these devices are firmly sutured in place between the skin and chest wall there is little chance of dangerous movement or dislodgment.
Nearly all other non-port vascular access catheters and devices do not contain metal and are MR Safe. These include Peripherally Inserted Central Catheter (PICC) lines, central venous catheters (CVCs), and tunneled catheters (e.g., Hickman, Broviac, Permacath, Leonard, and Groshong).
 "MR Unsafe" Arrow EZ-IO needle
"MR Unsafe" Arrow EZ-IO needle
The single exception is the ARROW® EZ-IO® intraosseous vascular access system declared MR Unsafe by its manufacturer (Teleflex) because it is made of 304 (ferromagnetic) stainless steel. The device is simply a short metal needle whose tip is placed in the marrow cavity of the tibia or other bone for infusion when peripheral vascular sites are unavailable. As their placement can be very tenuous (especially in children) and only slight movement can make them nonfunctional, their use in MRI is contraindicated. That said, in adults IO needles are literally screwed into the dense cortical bone and are usually solidly seated, making it unlikely they would be dislodged in a magnetic field. So I would personally be less concerned about the use in MRI if firmly implanted in older children or adults (but might wrap the site as a precaution).
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Blanco-Guzman MO. Implanted vascular access device options: a focused review on safety and outcomes. Transfusion 2018; 58:558-568. [DOI LINK]
Shellock FG, Shellock VJ. Vascular access ports and catheters: Ex vivo testing of ferromagnetism, heating, and artifacts associated with MR imaging. Magn Reson Imaging 1996; 14:443-447. [DOI LINK]
Blanco-Guzman MO. Implanted vascular access device options: a focused review on safety and outcomes. Transfusion 2018; 58:558-568. [DOI LINK]
Shellock FG, Shellock VJ. Vascular access ports and catheters: Ex vivo testing of ferromagnetism, heating, and artifacts associated with MR imaging. Magn Reson Imaging 1996; 14:443-447. [DOI LINK]
Related Questions
I heard Swan-Ganz catheters can melt in the MRI. Are any similar devices safe to scan?
I heard Swan-Ganz catheters can melt in the MRI. Are any similar devices safe to scan?