Frankly, I am not enthusiastic about the widespread clinical applicability of quantitative DCE at its present level of development. Looking critically at the existing literature, the reader will find significant site-to-site variability of measured parameters. When disparate tumors (such as lymphomas vs glioblastomas) are compared, statistically significant differences in variables like Ktrans and ve have been noted, but the overlap between groups makes these distinctions worthless in practice. In most of the brain tumor cases we have studied to date, quantitative DCE simply reinforces diagnostic probabilities obtainable by visual inspection of time-intensity contrast uptake curves or parameters obtainable by conventional, DSC, or DW imaging. If you do wish to believe, and want to see why some think quantitative DCE imaging has a bright future, read on. . .
The pathophysiological basis of DCE imaging lies in the (imperfect) concept that more malignant/aggressive neoplasms have greater degrees of neovascularity. To supply nutrients to their rapidly proliferating cells, blood flow and blood volumes typically increase. The fraction of tissue occupied by blood vessels and plasma vp may also increase. Tumor blood vessels have increased surface areas and are also inherently leaky. Thus their permeabiltity-surface area products are elevated, and we would expect their Ktrans values to be high as well. Additionally, malignant tumors often have regions of necrosis producing in an increased extracellular space fraction (ve) compared to more benign tumors.
One of the principal concepts driving oncologists to push for quantitative DCE imaging is its potential to predict and monitor tumor response. A number of new chemotherapeutic agents such as bevacizumab and cediranib are directed against vascular endothelial growth factor (VEGF). These agents are not directly cytotoxic against cancer cells, and hence do not result in immediate shrinkage of tumors. However, by reducing vascular permeability they affect Ktrans and vp, thus presenting a potentially quantifiable method to assess effectiveness of therapy. For similar reasons, DCE parameters hold promise to distinguish progression from pseudoprogression and recurrent tumor vs radionecrosis. Two of our cases showing application of DCE imaging to brain neoplasms after therapy are presented below.
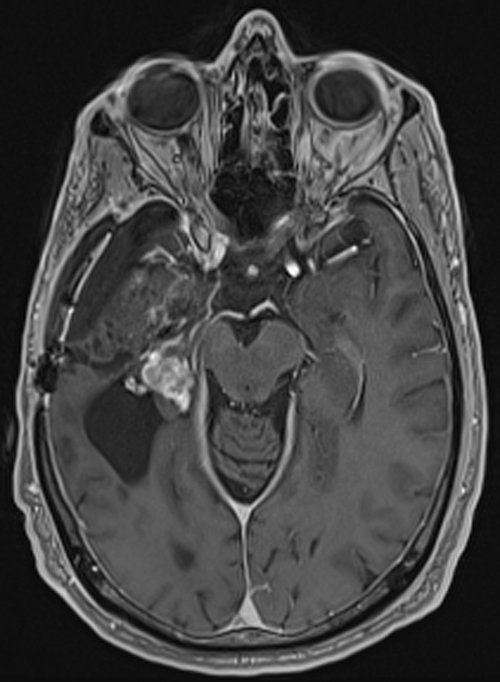
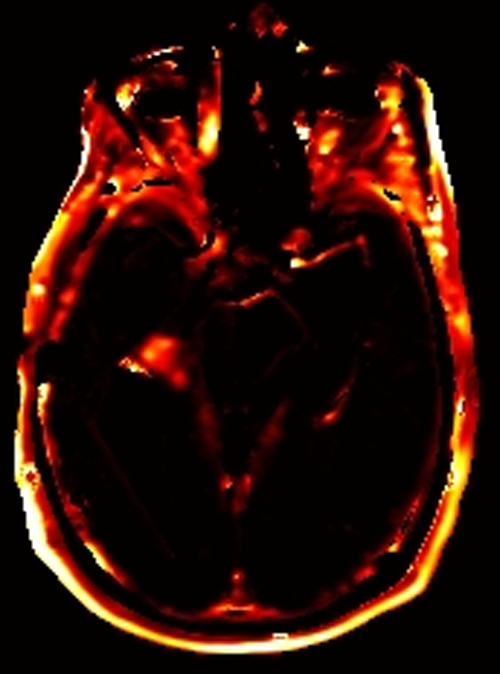
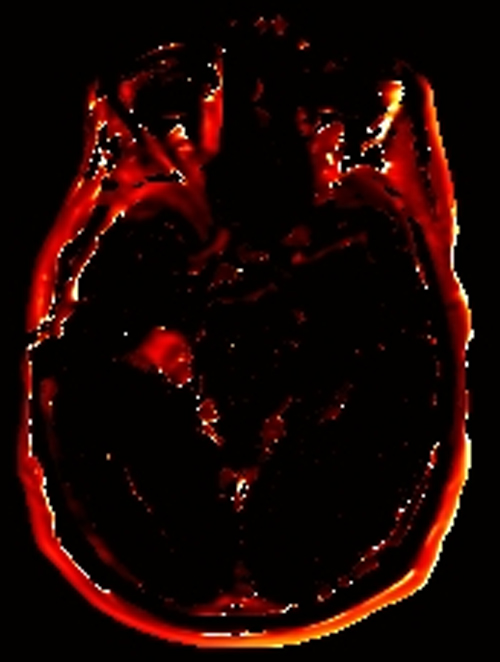
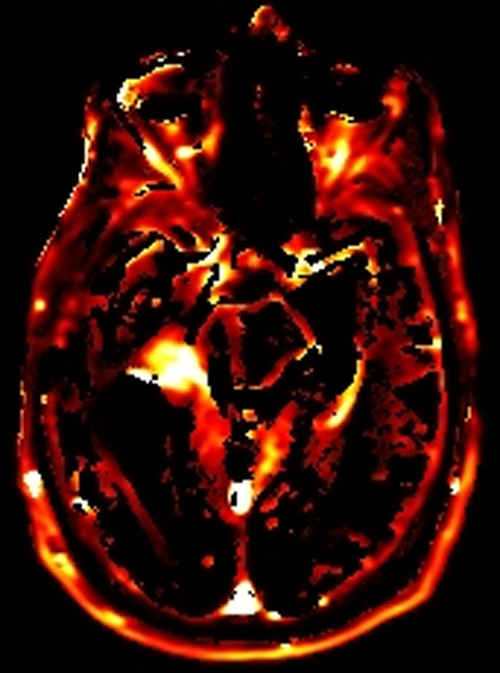
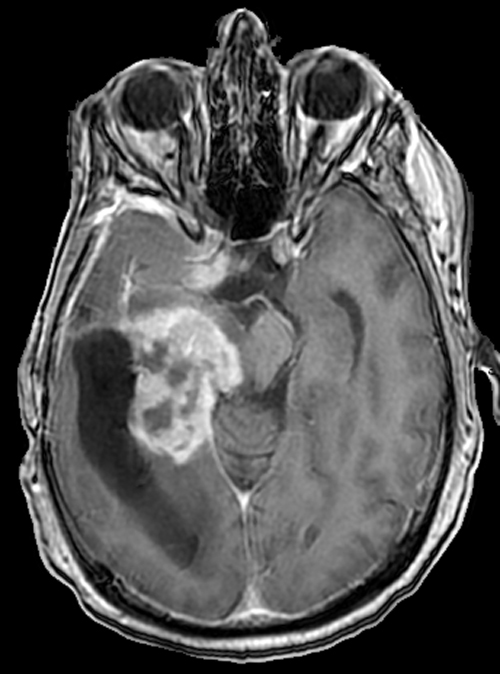
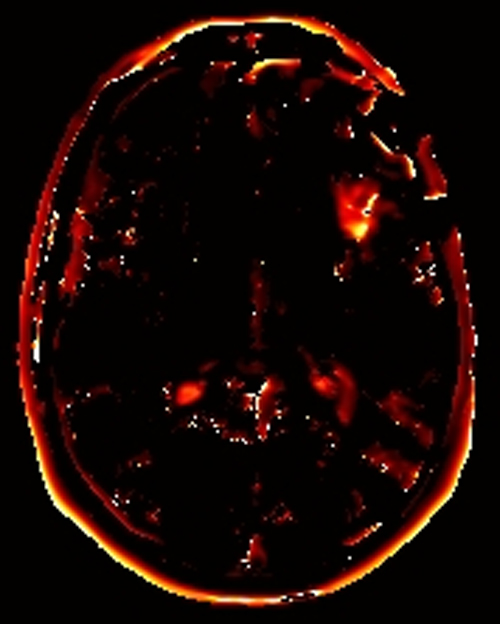
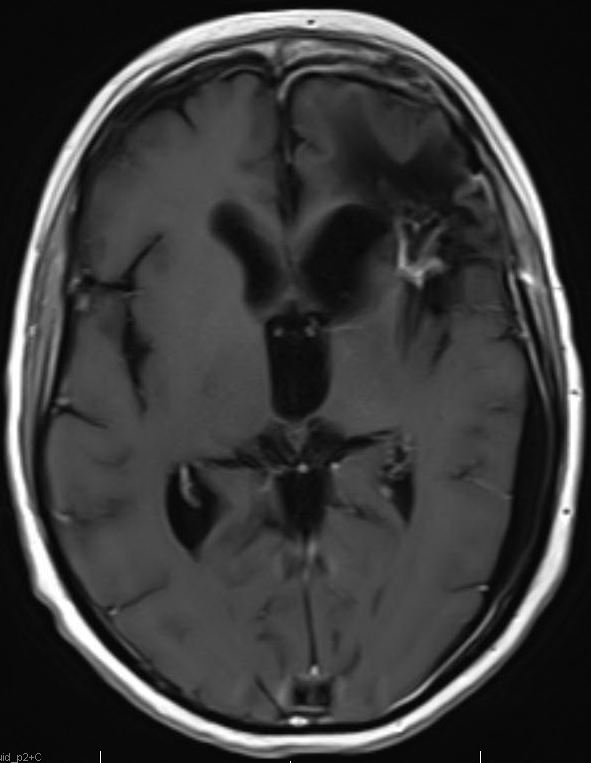
Above. DCE study positive/predictive for recurrent glioma. Contrast enhancement with elevations of Ktrans, ve, and vp. Follow-up at 10 months shows marked progression of tumor.
Below. Equivocal DCE study in a treated glioma. Contrast enhancement with marked elevation of Ktrans and ve noted, as well as a slight elevation of vp. Is this viable tumor? Follow-up 3 years later shows treatment related changes only, no tumor progression or recurrence.
Advanced Discussion (show/hide)»
Anzalone N, Castellano A, Cadioli M, et al. Brain gliomas: multicenter standardized assessment of dynamic contrast-enhanced and dynamic susceptibility contrast MR images. Radiology 2018; (in press)(recent paper showing good results using DCE and DSC to predict grade of brain tumors)
Beuzit L, Eliat P-A, Brun V, et al. Dynamic contrast-enhanced MRI: study of inter-software accuracy and reproducibility using simulated and clinical data. J Magn Reson Imaging 2016; 43:1288-1300. (Poor agreement and reproducibility testing 5 commercial DCE-MRI software packages).
Choi HS, KIm AH, Ahn SS, et al. Glioma grading capability: comparisons among parameters from dynamic contrast-enhanced MRI and ADC value on DWI. Korean J Radiol 2013; 14:487-92. (sounds great, but remember that many of the low-grade gliomas enhance weakly, if at all, so DCE parameters will reflect this.)
Chikui T, Obara M, Simonetti AW, et al. The principal (sp) of dynamic contrast enhanced MRI, the method of pharmacokinetic analysis, and its application in the head and neck region. Int J Dentistry 2012; doi:10.1155/2012/480659
Fatterpekar GM, Galheigo D, Naryana A, et al. Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum — use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. Am J Roentgenol 2012; 198:19-26. (acutally about DSC, not DCE, but gives an excellent description of the pathology of treatment-related changes in brain tumors).
Kickingereder P, Sahm F, Wiestler B, et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. AJNR Am J Neuroradiol 2014; 35:1503-1508. (some statistical differences in DCE parameters noted, but significant overlap between the two diseases in my opinion makes the technique worthless.)
Heye AK, Culling RD, del C Valdés Hernández M, et al. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage: Clinical 2014; 6:262-274. [DOI LINK]
Manning C, Stringer M, Dickie B, et al. Sources of systematic error in DCE-MRI estimation of low-level blood-brain barrier leakage. Magn Reson Med 2021; (in press) [DOI LINK] (Casts doubt on accuracy of Patlak model for measuring BBB disruption)
Zhang N, Zhang L, Qui B, et al. Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson imaging 2012; 36:355-363.