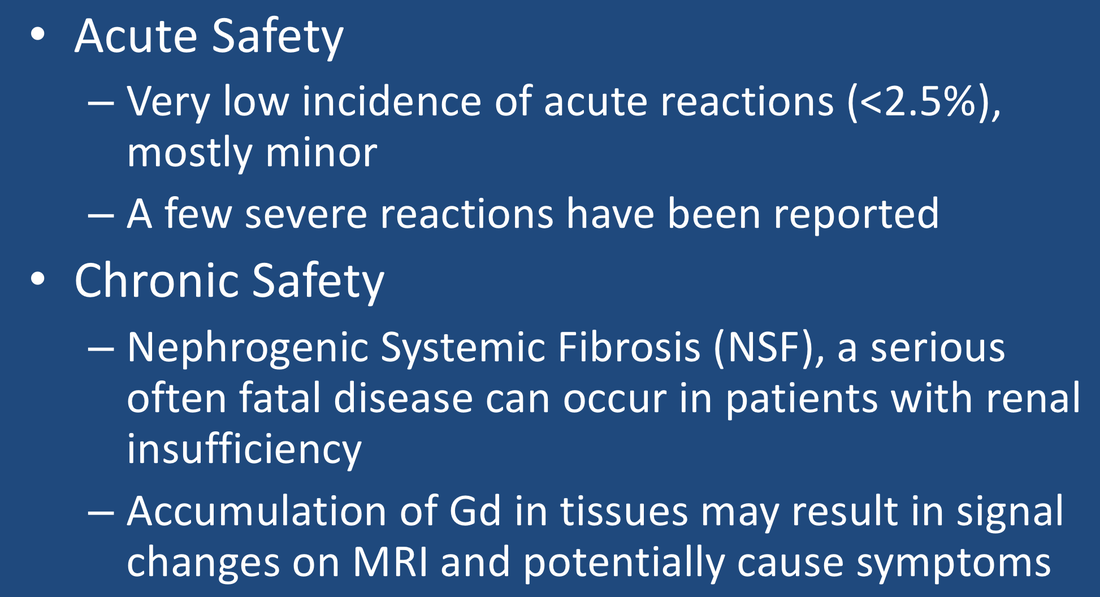
Compared to many other drugs gadolinium contrast agents possess a very low incidence (<2.5%) of acute adverse reactions. Nearly all of these reactions can be characterized as very mild, the most common being nausea, vomiting, headache, a metallic taste, injection site discomfort, warmth, paresthesias, and dizziness. Such rates are not too much higher than those occurring during placebo injection of saline and are about one-third as common as reaction rates recorded with nonionic iodine-based contrast media.
Immediate hypersensitivity reactions occur in approximately 1 in 1000 cases, usually within 1 hour of contrast administration. These are more likely in patients with allergies, asthma, and prior reaction to gadolinium contrast. The most common of these reactions are mild pruritus (itching) and urticaria (hives). Prior reaction to gadolinium contrast confers an 8-fold higher risk for a future reaction, often more severe than the first.
Moderately severe hypersensitivity reactions (including bronchospasm, laryngospasm, facial edema, tachycardia, arrhythmias, or widespread urticaria) occur in about 1 in 5000 cases. Seizures induced directly by MR contrast agents in epileptic patients have been reported, but are too rare to represent a contraindication for the use of these drugs in evaluating patients with seizures. Extremely rare reported reactions include acute pancreatitis, acute renal failure, and encephalopathy.
|
Worldwide, a number of severe anaphylactoid reactions to gadolinium-based MR contrast agents, including death, have been reported. The incidence of severe reactions is about 1 in 20,000 and the risk of death about 1 in 400,000.
|
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” – Paracelsus |
The above discussion describes general adverse physiologic and allergic-like events and does include special situations (including use in pregnancy, infancy, and renal insufficiency). These important topics each have devoted Q&A's.
Chronic Effects
The most famous of the chronic reactions to gadolinium contrast is Nephrogenic Systemic Fibrosis (NSF). This is a rare, progressive, and usually fatal disorder characterized by skin thickening, painful joint contractures, and fibrosis of multiple organs including the lungs, liver, muscles, and heart. Nearly all cases have occurred in patients with chronic severe renal insufficiency. First reported in 2006, several hundred patients have been diagnosed world-wide. Because of diligence by the greater radiology community, since 2012 full-blown NSF has now been essentially eliminated as an existing disease. However, gadolinium-containing plaques in the extremities have been reported and may represent a forme fruste of NSF.
Considerable recent interest has been directed to the potential for more subtle long-term effects due to gadolinium deposition in tissues. Increased T1 signal may be seen in the basal gangia and dentate nuclei of patients receiving multiple gadolinium injections secondary to gadolinium accumulation. Some neurological and pain syndromes have been reported by patients and the concept of gadolinium-deposition disease has been promulgated to explain these. This is a controversial subject and will be covered in a separate Q&A.
In conclusion, although the gadolinum-based MR contrast agents are extremely safe, they are not infinitely so. One should always remember that administration of any drug carries with it the risk of a life-threatening reaction and long-term risks of gadolinium deposition must be considered.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
ACR Committee on Drugs and Contrast Agents. ACR Manual on Contrast Agents, 2023. American College of Radiology, 2022.
Ahn YH, Kang DY, Park S-B, et al. Allergic-like hypersensitivity reactions to gadolinium-based contrast agents: an 8-year cohort study of 154539 patients. Radiology 2022; 303:329-336. [DOI LINK] (About a 4-fold risk of reaction to gadolinium contrast agents occurs in patients allergic to iodine-CT contrast, thought to represent just a general allergic sensitivity rather than true cross-reactivity.)
Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systemic review and meta-analysis. Radiology 2018; 286:471-482. (Surprisingly, Omniscan had the lowest rate of reactions, and macrocyclics had higher rates than linear agents)
Dillman JR, Ellis JH, Cohan RH, et al. Frequency and severity of acute allergic-like reactions to gadolinium-containing IV contrast media in children and adults. AJR Am J Roengenol 2007; 189:1533-1538.
Hunt CH, Hartman RP, Hesley GK. Frequency and severity of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009; 193:1124-1127.
Jung J-W, Kang H-R, Kim M-H, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012; 264:414-422.
Kanal E, Maravilla K, Rowley HA. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 2014; 35: 2215-2226.
McDonald JS, Hunt CH, Kolbe AB, et al. Acute adverse events following gadolinium-based contrast agent administration: a single-center retrospective study of 281945 injections. Radiology 2019; 292:620-627.
Ramalho J, Ramalho M, Jay M, et al. Gadolinium toxicity and treatment. Magn Reson Imaging 2016; 34:1394-8.
ACR Committee on Drugs and Contrast Agents. ACR Manual on Contrast Agents, 2023. American College of Radiology, 2022.
Ahn YH, Kang DY, Park S-B, et al. Allergic-like hypersensitivity reactions to gadolinium-based contrast agents: an 8-year cohort study of 154539 patients. Radiology 2022; 303:329-336. [DOI LINK] (About a 4-fold risk of reaction to gadolinium contrast agents occurs in patients allergic to iodine-CT contrast, thought to represent just a general allergic sensitivity rather than true cross-reactivity.)
Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systemic review and meta-analysis. Radiology 2018; 286:471-482. (Surprisingly, Omniscan had the lowest rate of reactions, and macrocyclics had higher rates than linear agents)
Dillman JR, Ellis JH, Cohan RH, et al. Frequency and severity of acute allergic-like reactions to gadolinium-containing IV contrast media in children and adults. AJR Am J Roengenol 2007; 189:1533-1538.
Hunt CH, Hartman RP, Hesley GK. Frequency and severity of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009; 193:1124-1127.
Jung J-W, Kang H-R, Kim M-H, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012; 264:414-422.
Kanal E, Maravilla K, Rowley HA. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 2014; 35: 2215-2226.
McDonald JS, Hunt CH, Kolbe AB, et al. Acute adverse events following gadolinium-based contrast agent administration: a single-center retrospective study of 281945 injections. Radiology 2019; 292:620-627.
Ramalho J, Ramalho M, Jay M, et al. Gadolinium toxicity and treatment. Magn Reson Imaging 2016; 34:1394-8.
Related Questions
What kind of informed consent to you give your patients receiving gadolinium? Do you tell them about NSF?
What kind of informed consent to you give your patients receiving gadolinium? Do you tell them about NSF?