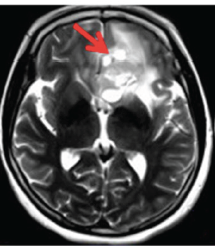
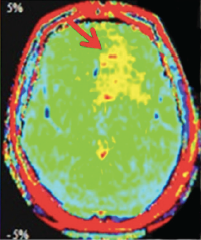
Amide Proton Transfer (APT) imaging is a relatively new method using off-resonance saturation pulses to detect mobile proteins and peptides in biological tissues. The premise underlying its potential utility is that certain diseases such as malignant tumors with high cellularity and processes that denature proteins (like multiple sclerosis) may exhibit elevated APT values.
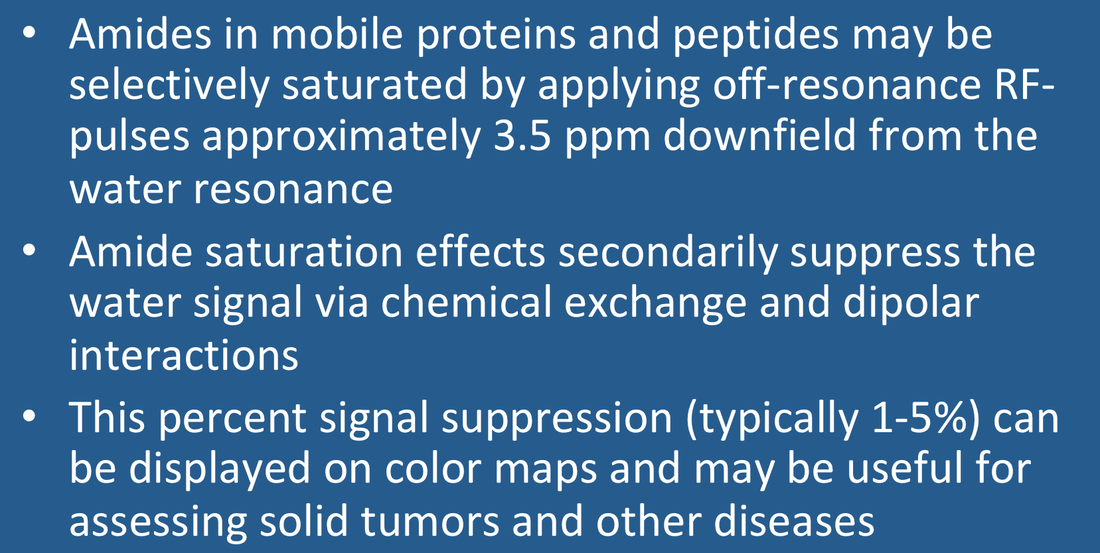
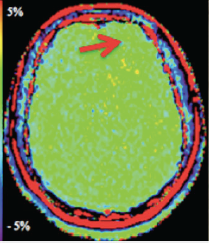
APT imaging of low versus high grade gliomas (From Bai et al 2017 under CC BY)

APT, like Magnetization Transfer (MT) imaging, is a subset of a more general class of methods known as Chemical Exchange Saturation Techniques (CEST). The basic concept is that proteins and other macromolecules can absorb RF energy at frequencies applied a few hundred to a few thousand Hz away from the pure water resonance. When such an "off-resonance" RF-pulse is applied, energy absorbed by macromolecules is then transferred via chemical exchange and dipolar interactions to the water resonance, lowering its signal. The degree of signal suppression will be greatest for tissues with high protein content and high rates of water-macromolecular interactions.
 An amide functional group is defined as a nitrogen (N) attached to a carbonyl (C=O) group. The R's may be hydrogens (H) or an alkane.
An amide functional group is defined as a nitrogen (N) attached to a carbonyl (C=O) group. The R's may be hydrogens (H) or an alkane.
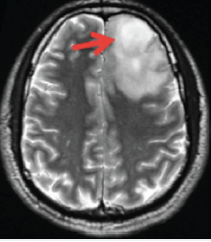
Although general magnetization transfer effects are broad and non-specific, it is possible to hone in on a group of mobile proteins and peptides known as amides, which exist at reasonable concentrations (~5-8 mM) and which can be selectively stimulated by off-resonance RF-pulses applied at approximately 3.5 ppm downfield from the water resonance (i.e., at δ ≈ 8.2 ppm). To isolate the effect of amide proton transfer from that of other background macromolecules, the APT pulse sequence uses 8-10 RF-pulses of slightly different off-resonance frequencies clustered near both +3.5 ppm (at δ ≈ 8.2) and −3.5 ppm (at δ ≈ 1.2) symmetrically on each side of the water resonance. After correction for Bo nonuniformities and measurement of baseline signal (So) in the absence of RF-pulses, APT suppression is calculated using subtraction on a pixel-by-pixel basis. Such APT values in tissues typically range between 0 and 5% and can be displayed as color maps overlaid on conventional images.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Bai Y, Lin Y, Zhang W, et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 2017; 8:5834-5842.
Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging 2017; 47:11-27.
Knutsson L, Xu J, Ahlgren A, van Zijl PCM. CEST, ASL and magnetization transfer contrast: how similar pulse sequences detect different phenomena. Magn Reson Med 2018; 1320-1340.
van de Ven K, Keupp J. Amide proton transfer weighted imaging: advancement in molecular tumor diagnosis. Philips White Paper Series, 2018:1-15. (excellent technical review with clinical examples)
van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 2011; 65:927-948.
Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reson 2013: 229:155-172.
Zhou J, Lal B, Wilson DA et al. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 2003; 50:1120-1126.
Zhou J, Payen J-F, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med 2003; 9:1085-1090.
Bai Y, Lin Y, Zhang W, et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 2017; 8:5834-5842.
Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging 2017; 47:11-27.
Knutsson L, Xu J, Ahlgren A, van Zijl PCM. CEST, ASL and magnetization transfer contrast: how similar pulse sequences detect different phenomena. Magn Reson Med 2018; 1320-1340.
van de Ven K, Keupp J. Amide proton transfer weighted imaging: advancement in molecular tumor diagnosis. Philips White Paper Series, 2018:1-15. (excellent technical review with clinical examples)
van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 2011; 65:927-948.
Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reson 2013: 229:155-172.
Zhou J, Lal B, Wilson DA et al. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 2003; 50:1120-1126.
Zhou J, Payen J-F, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med 2003; 9:1085-1090.
Related Questions
What is magnetization transfer?
How is contrast generated by magnetization transfer? Also, what are MTI, MTC, and MTR?
What is magnetization transfer?
How is contrast generated by magnetization transfer? Also, what are MTI, MTC, and MTR?