|
To allow reversible binding of oxygen (O2), the iron centers of deoxyhemoglobin must remain in the reduced, ferrous (Fe+2) state. Once hemoglobin has been removed from the high-oxygen environment of the circulation, however, various metabolic pathways (e.g., methemoglobin reductase) fail, allowing heme iron to become oxidized to the ferric (Fe+3) form, methemoglobin. As a result of this change in valence state, met-Hb is unable to bind oxygen. The transition from deoxy-Hb to met-Hb requires the loss of an electron from each iron center and rearrangement of electron spins in the 3d subshell. The end result is that met-Hb has 5 unpaired electrons, rendering it slightly more paramagnetic than deoxy-Hb (which has only 4 unpaired electrons). By comparison, gadolinium used for MR contrast agents has 7 unpaired electrons, the most of any element.
|
|
Also with the ferrous-ferric transition, the crevices in the globin subunits that previously allowed binding of oxygen are reshaped permitting access of water very close to the iron centers for the first time. The resulting complex is known as aquo-methemoglobin. This allows so-called inner sphere relaxation to occur, resulting in very short T1 values (and corresponding brightness on T1-weighted images). As long as met-Hb continues to be compartmentalized intracellularly, however, local magnetic susceptibility effects persist and the hematoma remains dark on T2/T2*-weighted images.
The subacute stage of hematoma formation is therefore distinguished chemically and radiologically by the presence and location of methemoglobin. The early subacute phase (illustrated below 2 days - 1 week) is characterized by intracellular met-Hb. The late subacute phase (1 week - 2 months, described in a later Q&A) is characterized by extracellular met-Hb.
|
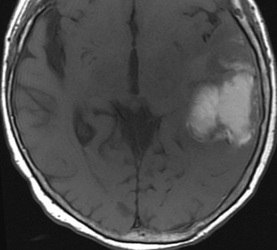
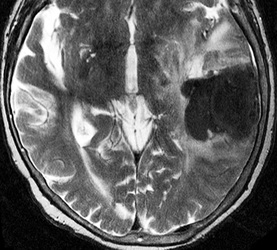
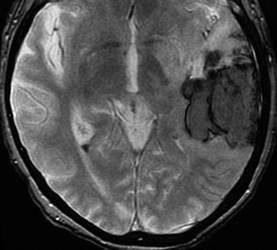
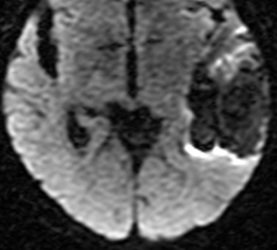
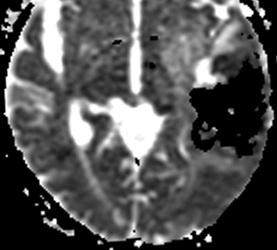
Below: MR images obtained about 3 days after hemorrhage illustrating prominent intracellular methemoglobin formation. Hematoma is hyperintense to brain on T1-weighted image (top left). Center of hematoma is markedly hypointense on T2-weighted image (top center). A halo of high signal edema in the surrounding brain is noted. SWI image (top right) shows loss of signal due to T2* dephasing from paramagnetic met-Hb confined to RBCs. Even more hypointensity is seen at periphery due to accumulation of ferritin and hemosiderin. Trace DW image (bottom left) and ADC map (bottom middle) both have dark centers, due to strong paramagnetic artifacts and the T2-blackout effect. Brighter susceptibility artifacts is present at periphery of Trace image.
Important Note: The discussion above primarily applies to higher field (≥ 0.5 T) systems where imaging findings are dominated by the paramagnetic effects of hemoglobin. At low and intermediate fields some important differences in imaging appearances are noted. See this Q&A for a more detailed analysis.
Advanced Discussion (show/hide)»
Methemoglobin can also be produced directly from oxyhemoglobin (oxy-Hb) without going through the deoxy-Hb intermediary. This occurs occasionally when O2 takes an extra electron with it when released from oxy-Hb. The loss of oxygen as this superoxide (O2•-) results in formation of methemoglobin with iron in the Fe+3 state. Spontaneous formation of Met-Hb naturally by this mechanism occurs in the normal circulation, but typically constitutes much less than 1% of total blood hemoglobin.
Methemoglobin may also accumulate in the peripheral blood as a pathologic condition, an entity known as methemoglobinemia. Methemoglobinemia may be congenital or acquired. Congenital causes include hemoglobin mutation (hemoglobin M) and deficiency of NADH methemoglobin reductase enzyme system. Acquired methemoglobinemia is usually drug-induced. Common offenders include benzocaine-based topical anesthetics, antibiotics (sulfa drugs, dapsone, chloroquine), and exogenous nitrites.
The electronic configuration of Fe in various forms of hemoglobin affects the location of the iron center with respect to the plane of the pyrrole ring and subsequently the stereochemistry of the molecule. In oxyhemoglobin the iron lies within the plane of the ring; in deoxyHb it is about 0.75Å above the plane; in metHb it is about 0.4Å out of plane. As the iron center is coordinated with His in all these forms, the displacement of iron acts as a lever that causes a distortion of the entire hemoglobin molecule. These distortions open and close crevices on the surface of the globin subunits allowing access of small molecules such as O2 or H2O to the iron centers.
In methemoglobin, water has access to the iron centers by these crevices for the first time. In the α subunits water molecules can approach within 2.2 Å but are held more tightly. In the β subunits water can only approach to within 2.5 A. However, the β sites allow greater exchange of water molecules in and out of distal pocket and thus contribute somewhat more to T1 relaxation.
Methemoglobin exists in both an acid and a base form, depending on whether H2O or OH− coordinates with the iron center. At normal physiological pH's, the acid form (H2O) predominates.
You may recall from general medicine that met-Hb is said to shift the oxygen-hemoglobin dissociation curve to the left. How can this be if met-Hb is unable to bind O2 at all?
The answer is that not all 4 heme irons in a given molecule are necessarily converted to the same Fe+3 state. Some of the subunits may remain in reduced (Fe+2) states and still able to bind oxygen. These intermediate forms are known as valence hybrids. The presence of at least one subunit in the Fe+3 state affects increases the oxygen affinity for the remaining ferrous hemes in the hemoglobin tetramer. The remaining hemes bind O2 more efficiently, but at the same time make them less able to release O2 to the tissues. This accounts for the left-shift of the oxygen-Hb dissociation curve.
Berman HM, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucleic Acids Res 2000; 28:235-242 at www.rcsb.org (A great site well known to biochemists with over 34,000 protein sequences and 3D viewers that allow you to make images).
Chevion M, Ilan YA, Samuni A, et al. Quaternary structure of methemoglobin. Pulse radiolysis study of the binding of oxygen to the valence hybrid. J Biol Chem 1979; 254:6370-6374.
Umbreit J. Methemoglobin--it's not just blue: a concise review. Am J Hematol 2007; 82:134-144.
Yi J, Thomas LM, Richter-Addo GB. Structure of human R-state aquomethemoglobin at 2.0 Å resolution. Acta Cryst 2011; F67:1-5.
What are the different forms of hemoglobin and why do they have different magnetic properties?
How does gadolinium cause relaxation?