|
Video Endoscopy Capsules
PillCam™ is a popular video capsule endoscopy device produced by Medtronic (Minneapolis, MN, USA). Several other medical equipment companies produce similar devices, including the following — CapsoCam™ (CapsoVision), ENDOCAPSULE (Olympus), OMOM (Jinshan), and MiroCam® (IntroMedic) — but the generic term "pillcam" has become ubiquitous when referring to these devices (akin to the use of the word "kleenex" for any tissue paper).
Video endoscopy capsules contain a small battery, camera, and transmitter circuitry. Some models are optimized for the small bowel or colon. The devices take thousands of pictures as they pass through the gastrointestinal tract before being eliminated in the feces. Occasionally the capsules will be retained in GI tract or a patient may present for MR imaging before the device has passed.
|
The official answer to "Can I scan someone with a retained PillCam™ or similar device?" must surely be "No", as all extant product literature considers them MR Unsafe on the basis that they might cause thermal or physical injury to the GI tract.
However, little if any testing of these devices in MRI has been conducted and several reports now exist of patients inadvertently scanned without any ill health effects. In one case, some "harm" occurred in that the imaging data was erased from the device. The capsules will also create a small-to-moderate sized susceptibility artifact. I therefore suspect that in the future, MR scanning with a PillCam™ in place will not be contraindicated, only discouraged.
As an accessory, Medtronic also sells Agile® and Patency® capsules consisting of a small RFID chip surrounded by a dissolvable core. These may be used in patients with suspected strictures demonstrate patency of the gastrointestinal tract prior to ingesting the PillCam™ itself. The Agile® and Patency® capsules are considered MR Unsafe, not because of any danger to the patient but because the RFID chip could malfunction in the MR field.
Other Capsule Devices
In addition to the PillCam™ family and their competitors, several other less common ingested capsule devices should be mentioned. For the same reasons as the PillCam™, all of the following are also considered MR Unsafe:
|
Core Temperature Monitoring Capsules
These single-use, ingestible capsules measure core body temperature (during the 1-5 day period they are within the body) and may be useful to monitor athletes and persons working in high-temperature environments. Several brands are commercially available: CorTemp™ (HQ Inc), VitalSense™ (Mini Mitter), myTemp™, and e-Celsius™ (BodyCap). In addition to the swallowed capsule, some systems have dermal patch receivers that are also MR Unsafe.
|
|
pH and Pressure Monitoring Capsules
The Bravo™ Esophageal Monitoring Capsule (Medtronic) measures pH and pressure in the distal esophagus. Placed endoscopically and held by suction, the capsule stays attached to the esophagus for several days, then spontaneously detaches to pass out through the GI tract. To ensure the capsule has been eliminated from the body, Medtronic warns that MRI should not be performed within 30 days of its placement.
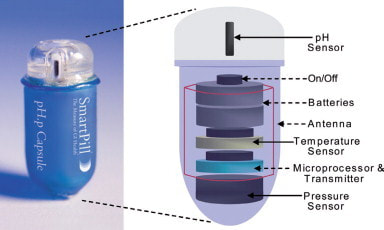
The SmartPill™ (Medtronic) provides pH, pressure, and position data on its transit through the GI tract. The device is marketed as part of a system for quantitative assessment of gastro-intestinal motility.
|
Drug Delivery Capsules
|
Although not in wide use, these devices represent a new class of ingestible capsules that hold, monitor, and release medications into the GI tract. They may also contain pH, location, and temperature sensors. Examples include the Abilify MyCite System (Proteus/ Otsuka), the ID-Cap System (etectRx), and the IntelliCap® (Medimetrics/Philips). MRI should not be performed with these in place.
|
References
Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 2016; 22:9898-9908. [DOI LINK]
Kalantar-zadeh K, Ha N, Ou JZ, Berean KJ. Ingestible sensors. ACS Sens 2017; 2:468-483. [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials—Part 1: gastrointestinal and vascular devices and materials. Diagn Interv Radiol 2020; 26:101–110. [DOI LINK]
Shamsudhin N, Zverev VI, Keller H, et al. Magnetically guided capsule endoscopy. Med Phys 2017; 44(8):e91-e111.
Waltz E. Drugs go wireless. Nature Biotech 2016; 34:15-18.
Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 2016; 22:9898-9908. [DOI LINK]
Kalantar-zadeh K, Ha N, Ou JZ, Berean KJ. Ingestible sensors. ACS Sens 2017; 2:468-483. [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials—Part 1: gastrointestinal and vascular devices and materials. Diagn Interv Radiol 2020; 26:101–110. [DOI LINK]
Shamsudhin N, Zverev VI, Keller H, et al. Magnetically guided capsule endoscopy. Med Phys 2017; 44(8):e91-e111.
Waltz E. Drugs go wireless. Nature Biotech 2016; 34:15-18.
Related Questions
What other GI devices warrant concern for MRI screening?
I assume that gastric pacemakers are forbidden in MRI. Correct?
What other GI devices warrant concern for MRI screening?
I assume that gastric pacemakers are forbidden in MRI. Correct?