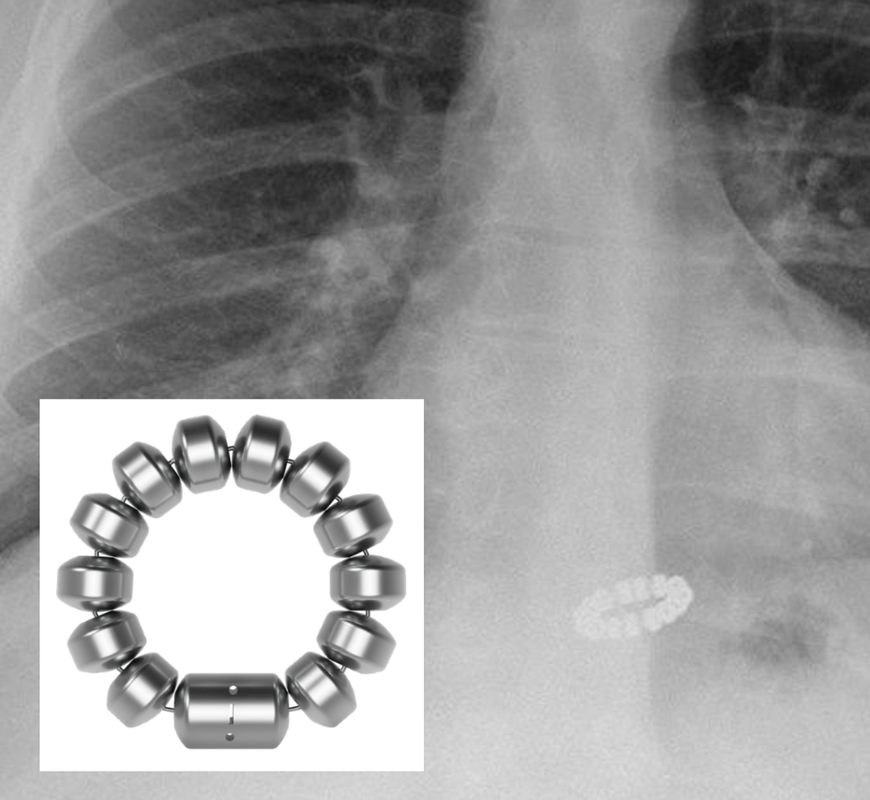
"MR Conditional" Instinct® endoclip
"MR Conditional" Instinct® endoclip(Cook Medical)
Endoscopy Clips
Metallic clips used in endoscopy, also known as endoclips, are placed along the walls of the gastrointestinal tract for hemostasis, closing perforations, and marking lesions. Produced by multiple companies (e.g., Boston Scientific, Cook, Olympus), only a small handful have ever been declared MR Unsafe, and to my knowledge only one of these is still in production (the QuickClip2 Long model HX201LR-135L by Olympus). All remaining clips on the market are made of titanium, tantalum, and non- or weakly- ferromagnetic stainless steel and are labeled MR Conditional. Notwithstanding this designation, a single case report describes an MR Conditional stainless steel Instinct® endoclip (Cook) successfully placed to control hemorrhage that became dislodged 5 days later immediately after an MR scan with massive hemorrhage and death.
Metallic clips used in endoscopy, also known as endoclips, are placed along the walls of the gastrointestinal tract for hemostasis, closing perforations, and marking lesions. Produced by multiple companies (e.g., Boston Scientific, Cook, Olympus), only a small handful have ever been declared MR Unsafe, and to my knowledge only one of these is still in production (the QuickClip2 Long model HX201LR-135L by Olympus). All remaining clips on the market are made of titanium, tantalum, and non- or weakly- ferromagnetic stainless steel and are labeled MR Conditional. Notwithstanding this designation, a single case report describes an MR Conditional stainless steel Instinct® endoclip (Cook) successfully placed to control hemorrhage that became dislodged 5 days later immediately after an MR scan with massive hemorrhage and death.
Enteric Tubes
 "MR unsafe" McClean-Ring Feeding Tube
"MR unsafe" McClean-Ring Feeding Tube(Cook Medical)
Orogastric, nasogastric, and transcutaneous gastrointestinal tubes containing no metal components are by definition MR Safe. Many tubes designed for feeding purposes have a weight in their distal portions, typically made of nonferromagnetic stainless steel or tungsten. These generally present no concern and nearly all are labeled MR Conditional. Even the unique Kangaroo™ Feeding Tube with IRIS Technology (it has a mini-camera at its distal end to assist with placement) is rated MR Conditional. Some devices, such as the ActiFlo Indwelling Bowel Catheter (Hollister) and the DigniShield™ Stool Management System (Bard), are what I consider "trivially MR conditional", because they contain a small metallic spring in a valve far outside the patient requiring only that the bag and distal catheter be secured to the table I am aware of only one currently manufactured enteric tube considered to be MR Unsafe — the McClean-Ring Feeding Tube (Cook), pictured above, which has moderately large stainless steel wrappings at its tip. Regardless of the catheter safety rating, however, always remember to remove the metal guiding stylet before MRI!
|
A unique device in this category is the Edi Catheter (Maquet). This nasogastric or orogastric tube contains a wire and sensor for measuring Electrical activity of the diaphragm. It is used in association with Neurally Adjusted Ventilatory Assistance (NAVA), a technique where the ventilator is triggered by the patient's own respiratory effort (measured by the diaphragm's electrical activity). Closely resembling a normal NG tube (in fact, it can also be used for suction or feeding), this device is considered MR Unsafe.
Stents
|
Stents are tube-like implants often in the form of a mesh designed to keep open the lumens of various anatomic structures affected by tumors, scarring, or other diseases. In gastroenterology, stents may be placed inside the visceral tract (esophagus, stomach, bowel) or within ducts (biliary or pancreatic). While ductal stents are commonly made of semi-rigid plastic (polyethylene), nearly all visceral tract tubes are self-expandible and made of metal (titanium, Nitinol, Elgiloy, or stainless steel) often covered by plastic or a bioabsorbable material.
 "MR Unsafe" Boubella Esophageal Stent (Ella-CS)
"MR Unsafe" Boubella Esophageal Stent (Ella-CS)
The vast majority of GI tract stents are MR Conditional , which generally limits field strength, SAR, and spatial gradient fields, but in some cases also includes a requirement to wait 6-8 weeks post implantation before scanning. There are two types of stents, both esophageal, that are known to be either MR Unsafe or "MR Untested":
- Esophageal Z-Stents® (Cook): About a dozen of these stainless steel models are still listed on the manufacturer's website with the statement "MR compatibility...has not been established."
- FerX-ELLA Esophageal Stent BOUBELLA (Ella-CS, Czech Rep): Although Ella's other esophageal stents are MR Conditional this particular model contains ferromagnetic stainless steel and is MR Unsafe. This stent never received FDA approval in the US, but is available throughout Europe.
|
Magnetic Rings
The LINX® Reflux Management System (Johnson & Johnson) consists of a ring of magnets surrounding the gastroesophageal junction placed by endoscopic surgery for treating reflux. Devices implanted before May 2015 are MR Conditional at 0.7T, while newer models are Conditional at 1.5T. A nearly identical device, the FENIX® Continence Restoration System, was placed around the anal sphincter to treat fecal incontinence. Although production of the FENIX® was discontinued in 2017, it is a lifetime implant that may occasionally be encountered in patients presenting for MRI.
|
|
Bariatric Devices
Adjustable Gastric Bands, typified by the branded LAP-BAND® (Apollo Endosurgery) and REALIZE (Ethicon Endosurgery) devices, are among the most widely used implants in bariatric surgery. Placed around the upper stomach they reduce the amount of food the stomach can hold. The size of the opening is gradually reduced over time using adjustments or fills. The filling ports of several models contain metallic parts, making these MR Conditional.
Gastric Balloons, placed endoscopically, are all made of non-metallic materials and are MR Safe. |
Some additional newer and less commonly used bariatric devices include:
- AspireAssist Aspiration Therapy (Aspire Bariatrics), a percutaneous external drainage system from the stomach made of plastic and silicone that is MR Safe
- Transpyloric Shuttle (TPS) (Baronova), a system with two bulb-like balloons, the smaller one of which slides into the duodenum when the stomach is filled. Composed mostly of silicone, the TPS has a small amount of internal metal making it MR Conditional.
- Incisionless Anastamotic System (IMAS) (GI Windows) creates an internal diversion between the proximal jejunum and distal ileum using a pair of self-assembling magnets delivered through an endoscope. The device is MR Unsafe while the magnetic rings remain attached. However, they are eventually self-eliminated in the stool, at which point no MR safety concerns exist.
Artificial Bowel Sphincters
 "MR Conditional" AMI Anal Soft Band System®
"MR Conditional" AMI Anal Soft Band System®
In addition to the discontinued magnetic FENIX® System described above, several other artificial bowel sphincters have been developed for treatment of fecal incontinence over the last ~20 years. Two of these, the Acitcon® Neosphincter and the Prostatic Anal Sphincter (PAS®), are no longer available, but the Anal Soft Band System® (AMI, Austria) is still in production. These systems are all share a similar design relying on fluid within a reservoir to be pumped into and out of a soft anal cuff. All three devices are made predominately of silicone, but have metallic springs in the valve mechanism as well as a titanium filling ports, making them technically MR Conditional (but reasonably safe to scan) at fields up to 3.0T.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Behrang A. Edi Catheter (NOT MRI SAFE). RoentgenRayReader.Blogspot.com 2018. (image above from this reference under CC BY)
Bonin EA, Verschoor B, Silva FH, et al. Stents in gastrointestinal disease. IntechOpen, 2019 published online. [DOI LINK]
Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc 2017; 50:11-16. [DOI LINK]
Fattorini E, Brusa T, Fingret C, et al. Artificial muscle devices: innovations and prospects for fecal incontinence treatment. Ann Biomed Eng 2016; 44:1355-1369. [DOI LINK]
Kurt M, Posul E, Tekelioglu V, et al. Are MR compatible hemoclips safe after control of hemostasis? Endoscopy 2014; 46(suppl 1):#471. (case report of a patient who died from massive hemorrhage after presumed displacement of an MR Conditional endoclip) [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials—Part 1: gastrointestinal and vascular devices and materials. Diagn Interv Radiol 2020; 26:101–110. [DOI LINK]
Shin DY, Park S, Kim A, et al. Compatibility of endoclips in the gastrointestinal tract with magnetic resonance imaging. Sci Reports 2020; 10:16357. [DOI LINK]
Sonavane SK, Menias CO, Kantawala KP, Shanbhogue AK, et al. Laparoscopic adjustable gastric banding: what radiologists need to know. RadioGraphics 2012; 32:1161-1178. [DOI LINK]
Zubaidi AM. Artificial bowel sphincters for severe fecal incontinence. Are they a solution? Saudi Med J 2010; 31:965-973.
Behrang A. Edi Catheter (NOT MRI SAFE). RoentgenRayReader.Blogspot.com 2018. (image above from this reference under CC BY)
Bonin EA, Verschoor B, Silva FH, et al. Stents in gastrointestinal disease. IntechOpen, 2019 published online. [DOI LINK]
Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc 2017; 50:11-16. [DOI LINK]
Fattorini E, Brusa T, Fingret C, et al. Artificial muscle devices: innovations and prospects for fecal incontinence treatment. Ann Biomed Eng 2016; 44:1355-1369. [DOI LINK]
Kurt M, Posul E, Tekelioglu V, et al. Are MR compatible hemoclips safe after control of hemostasis? Endoscopy 2014; 46(suppl 1):#471. (case report of a patient who died from massive hemorrhage after presumed displacement of an MR Conditional endoclip) [DOI LINK]
Mathew RP, Sam M, Alexander T, et al. Abdominal and pelvic radiographs of medical devices and materials—Part 1: gastrointestinal and vascular devices and materials. Diagn Interv Radiol 2020; 26:101–110. [DOI LINK]
Shin DY, Park S, Kim A, et al. Compatibility of endoclips in the gastrointestinal tract with magnetic resonance imaging. Sci Reports 2020; 10:16357. [DOI LINK]
Sonavane SK, Menias CO, Kantawala KP, Shanbhogue AK, et al. Laparoscopic adjustable gastric banding: what radiologists need to know. RadioGraphics 2012; 32:1161-1178. [DOI LINK]
Zubaidi AM. Artificial bowel sphincters for severe fecal incontinence. Are they a solution? Saudi Med J 2010; 31:965-973.
Related Questions
Can I scan a patient with a retained PillCam™?
I assume that gastric pacemakers are forbidden in MRI. Correct?
Can I scan a patient with a retained PillCam™?
I assume that gastric pacemakers are forbidden in MRI. Correct?