Phosphorus (P) is the second most commonly used nucleus for biological MRS studies. Interest in phosphorus derives from the unique information it provides, including: (1) the composition of cell membranes; (2) bioenergetics and oxidative phosphorylation; and 3) intracellular pH and magnesium levels.
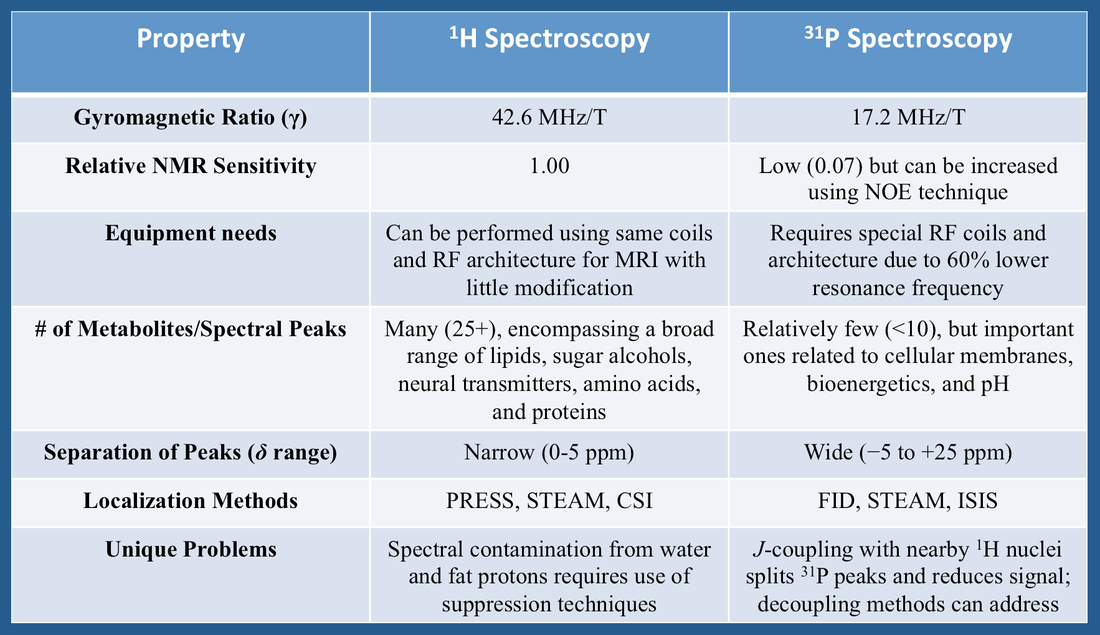
Phosphorus has only one naturally occurring stable isotope, ³¹P, which like ¹H has spin = ½ and thus two discrete energy states. The gyromagnetic ratio (γ) of ³¹P is 17.2 MHz/T, resulting in a resonance frequency about 60% lower than that of ¹H. Unlike ¹H NMR where the same radiofrequency (RF) front-end architecture can be used for both spectroscopy and imaging, ³¹P requires a separate set of RF coils and amplifiers tuned to this lower resonance frequency.
The nuclear sensitivity and concentration of ³¹P metabolites is also significantly lower than for ¹H, resulting in a weaker signal. Nevertheless, it is still relatively easy to obtain good quality phosphorus spectra with relatively sharp peaks, even at 1.5T. Special techniques such as Nuclear Overhauser Enhancement (NOE) can also be employed to increase ³¹P signal-to-noise.
The nuclear sensitivity and concentration of ³¹P metabolites is also significantly lower than for ¹H, resulting in a weaker signal. Nevertheless, it is still relatively easy to obtain good quality phosphorus spectra with relatively sharp peaks, even at 1.5T. Special techniques such as Nuclear Overhauser Enhancement (NOE) can also be employed to increase ³¹P signal-to-noise.
Compared to ¹H, the number of uniquely identifiable spectral peaks for ³¹P is relatively small (<10). Additionally, the chemical shift (δ) range of common phosphorus metabolites exceeds 25 ppm (about 6-fold larger than the range for ¹H), meaning that ³¹P peaks are more widely separated.
Finally, competing resonances from water and fat protons that contaminate ¹H spectra pose no problem for ³¹P spectroscopy. However, scalar (J)-coupling interactions between nearby ¹H and ³¹P nuclei do occur, splitting the spectral lines of phosphorus metabolites and reducing their intensities. To counteract this effect decoupling is commonly performed, in which RF-pulses tuned to the ¹H nuclei are applied during MR signal acquisition.
A summary of some differences between ¹H and ³¹P MR spectroscopy is provided in the table below.
Advanced Discussion (show/hide)»
Even though ¹H spectroscopy now dominates the MR landscape, in the "early years" (i.e., the 1980's) much more interest was focused on ³¹P than ¹H. In fact, the very first human MRS study and MRS of a disease were performed using ³¹P (see references below).
References
Andrade CS, Otaduy MCG, Park EJ, Leite CC. Phosphorus-31 MR spectroscopy of the human brain: technical aspects and biomedical applications. Int J Cur Res Rev 2014; 6:41-54.
Cresshull ID, Gordon RE, Hanley PE, et al. Localization of metabolites in animal and human tissue using ³¹P topical magnetic resonance. Bull Magnet Reson 1980; 2:426. (First MRS spectrum recorded in a human forearm.)
Li CW, Negendank WG, Murphy-Boesch J, et al. Molar quantitation of hepatic metabolites in vivo in proton-decoupled, nuclear Overhauser effect enhanced ³¹P NMR spectra localized by three-dimensional chemical shift imaging. NMR Biomed 1996; 9:141-155.
Ross BD, Radda GK, Gadian DG, et al. Examination of a case of suspected McArdle's syndrome by ³¹P nuclear magnetic resonance. N Engl J Med 1981; 304:1338-1342. (First application of MRS to a human disease.)
ten Hove M, Neubauer S. Evaluating metabolic changes in heart disease by magnetic resonance spectrosopy. Heart Metab 2006; 32:18-21.
Andrade CS, Otaduy MCG, Park EJ, Leite CC. Phosphorus-31 MR spectroscopy of the human brain: technical aspects and biomedical applications. Int J Cur Res Rev 2014; 6:41-54.
Cresshull ID, Gordon RE, Hanley PE, et al. Localization of metabolites in animal and human tissue using ³¹P topical magnetic resonance. Bull Magnet Reson 1980; 2:426. (First MRS spectrum recorded in a human forearm.)
Li CW, Negendank WG, Murphy-Boesch J, et al. Molar quantitation of hepatic metabolites in vivo in proton-decoupled, nuclear Overhauser effect enhanced ³¹P NMR spectra localized by three-dimensional chemical shift imaging. NMR Biomed 1996; 9:141-155.
Ross BD, Radda GK, Gadian DG, et al. Examination of a case of suspected McArdle's syndrome by ³¹P nuclear magnetic resonance. N Engl J Med 1981; 304:1338-1342. (First application of MRS to a human disease.)
ten Hove M, Neubauer S. Evaluating metabolic changes in heart disease by magnetic resonance spectrosopy. Heart Metab 2006; 32:18-21.
Related Questions
What is decoupling? Is it required for phosphorus spectroscopy?
Why do some spectra split into smaller peaks while others do not?
What must you do differently to perform ³¹P spectroscopy in lieu of ¹H spectroscopy?
What is NOE? How and when should it be used for phosphorus MRS?
What peaks are seen in the ³¹P spectrum and what do they mean?
What is decoupling? Is it required for phosphorus spectroscopy?
Why do some spectra split into smaller peaks while others do not?
What must you do differently to perform ³¹P spectroscopy in lieu of ¹H spectroscopy?
What is NOE? How and when should it be used for phosphorus MRS?
What peaks are seen in the ³¹P spectrum and what do they mean?