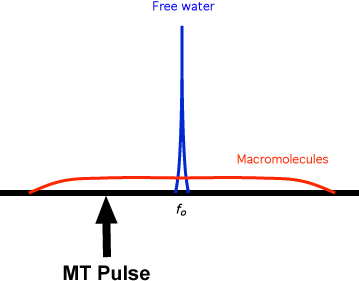
"Free" water is largely unstructured except by transient hydrogen bonding to similar molecules. Its molecules rotate very rapidly, most of which are ineffective at producing relaxation. Both the T1 and T2 of "free" water are very long. Only a very narrow range of frequencies (<0-100 Hz) near the Larmor frequency can be used to excite this pool to resonance.
The ¹H nuclei in macromolecules, by contrast, have highly restricted motions. They are subjected to static and low frequency magnetic fields from neighboring nuclei and paramagnetic ions and have very short T2 values. The local magnetic fields experienced by these nuclei may vary by up to 1 mT (10 G), representing a range of resonant frequencies offset from the Larmor frequency at 1T in the range of ± 40 kHz.
The T2 values of macromolecules are so short, in fact, that the direct signal from them decays so rapidly that they are not able to be directly recorded in routine MRI. Macromolecular ¹H nuclei do have important indirect effects, however, and significantly modulate the signal from water protons.
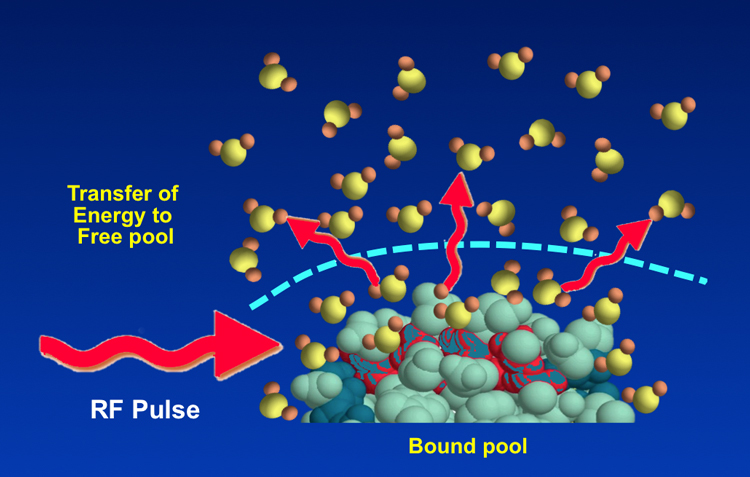
"Bound" water consists of a few layers of ¹H2O molecules closely associated with the surface of macromolecules. Water in this "hydration layer" is moderately structured and has restricted motion because of hydrogen bonding with sites on macromolecules. Thus, like macromolecules it has very short T2 values and a broad range of resonant frequencies. "Bound" water may undergo both dipole-dipole cross-relaxation as well as chemical exchange with macromolecular ¹H nuclei. "Bound" water also interfaces with "free" water on its outer surface, so it acts as a conduit for for the transfer of magnetization between the macromolecular and free water pools.
Because of this close relationship, "bound" water and macromolecules together are sometimes referred to as the "bound pool" while "free" water is called simply the "free pool".
In routine MRI a radiofrequency (RF) rotating magnetic field is applied at the Larmor (resonance) frequency with energy absorption primarily by free water protons. These excited ¹H nuclei then begin a T1 relaxation process where they release this absorbed energy to the "lattice". In this case the "lattice" includes unexcited nuclei in all three pools — free water, bound water, and macromolecules. This energy transfer occurs through several previously described mechanisms, especially dipole-dipole and chemical exchange interactions. The shifting of energy between pools is known as magnetization transfer.
Although we usually visualize the transfer of magnetization as free water → bound water → macromolecules, the process can proceed in the opposite direction. If we were to selectively deposit energy into the macromolecular pool (without affecting the water pool) using a specially designed RF pulse (called the MT Pulse), the free water nuclei could serve as the final "lattice" or reservoir to disperse the injected energy.
|
In clinical MRI MT pulses are used prior to certain sequences to improve contrast especially in MR angiography. Although on-resonance multinomial pulses can be used, the common method of RF excitation is to apply an MT pulse with a bandwidth of a few hundred hertz and center frequency shifted from the water resonance by 1000 to 25,000 Hz. This is typically followed by gradient spoiling to avoid interference patterns with the next radiofrequency (RF) pulse. This off-resonance pulse has sufficient power to saturate protons in the immobile pool without directly affecting those in free water. Following this MT saturation pulse, a conventional MRA or other pulse sequence is performed.
|
Advanced Discussion (show/hide)»
In the older NMR literature the term spin diffusion is sometimes used to refer to the same concept as magnetization transfer. This terminology is confusing because it is not the same as molecular diffusion which is a separate phenomenon.
The relative contributions of dipole-dipole interactions and chemical exchange to the magnetization transfer phenomenon are still being worked out. The current best evidence is that chemical exchange plays no more than a minor role in this process for most biological systems.
When an off-frequency MT pulse is applied to the system to saturate the bound pool, there is some obligatory spin-lock relaxation effects on the free water pool that contributes to its partial saturation. See the Dyke Award-winning paper by John Ulmer in the reference list for a more complete discussion of this.
de Boer RW. Magnetization transfer contrast. Part 1: MR Physics. Philips Medical Systems MedicaMundi 1995;40:64-73.
de Boer RW. Magnetization transfer contrast. Part 2: Clinical applications. Philips Medical Systems MedicaMundi 1995;40:74-83.
Edzes HT, Samulski ET. Cross-relaxation and spin diffusion in the proton NMR of hydrated collagen. Nature 1977;265:521-523. (A famous paper first showing how the interaction between water and proteins affects relaxation times.)
Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed 2001;14:57-64.
Knutsson L, Xu J, Ahlgren A, van Zijl PCM. CEST, ASL and magnetization transfer contrast: how similar pulse sequences detect different phenomena. Magn Reson Med 2018; 1320-1340.
Ulmer JL, Mathews VP, Hamilton CA, Elster AD, Moran PR. Magnetization transfer or spin-lock? An investigation of off-resonance saturation pulse imaging with varying frequency offsets. AJNR Am J Neuroradiol 1996; 17:805-819.
Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation In vivo. Magn Reson Med 1989; 10: 135-144.
How does the presence of macromolecules affect T1 and T2?
How is contrast generated by magnetization transfer? Also, what are MTI, MTC, and MTR?