Measurement of intracranial pressure (ICP) is a critical part of neurosurgical practice, especially for treatment of patients with traumatic head injury. As described in a prior Q&A, extraventricular drainage (EVD) systems are commonly used for this purpose and constitute the "gold standard" for ICP measurement (if not kinked or partially occluded). The current Q&A deals with electronic ICP measurement devices that utilize small catheters (probes) placed through the skull connected to bedside monitors. Many variations and configurations are possible:
|
About a half-dozen companies manufacture implantable ICP devices worldwide. Three basic transducer (sensing element) designs are used:
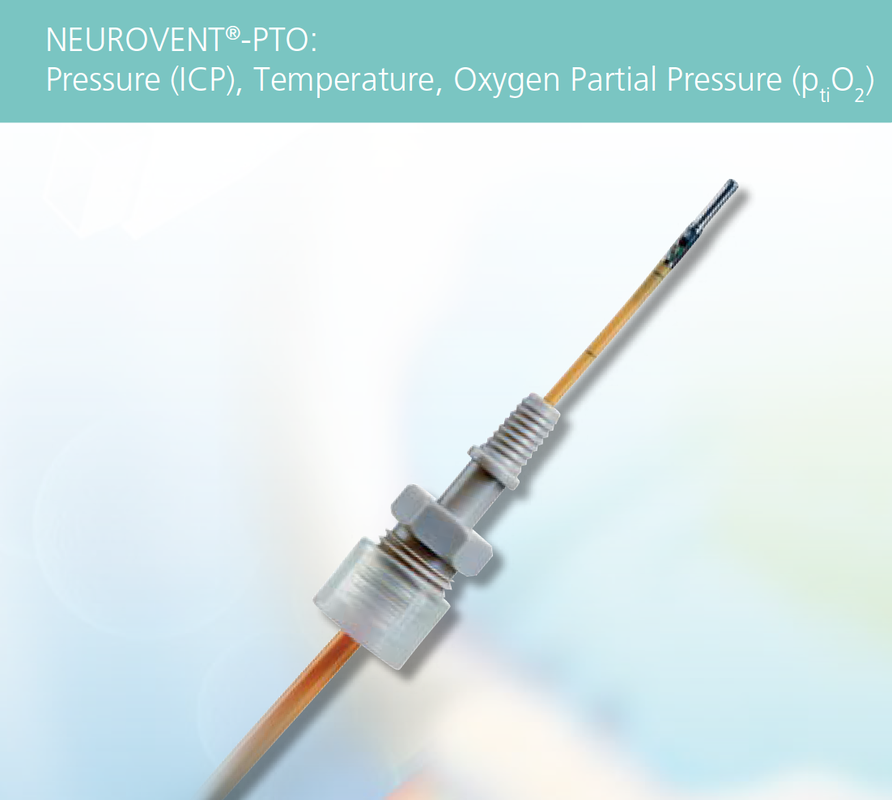
- Piezoelectric strain gauges are the most common ICP type, exemplified by the Codman® CereLink™ (Integra LifeSciences), the Neurovent® (Raumedic), and the Pressio® (Sophysa). The piezoelectic transducer lies at the tip of the nylon catheter in direct contact with the brain or CSF, which when deflected by pressure change generates a current. An electrical adaptor is at the end which connects to an external monitor. Nearly all of these devices are MR Conditional.
- Pneumatic sensors have a tiny balloon near the tip of the catheter, that when filled with air, transmits pressure waves to a diaphragm where both ICP and brain compliance can be measured. Examples include the Spiegelberg ICP Catheter (Spiegelberg), the ICB/T Pressure Transducer (Gaeltec), and the Hummingbird® (IRRAS). Nearly all of these devices are MR Conditional.
- Fiberoptic transducers employ a fiber optic cable to transmit light toward a displaceable mirror that moves in response to changes in ICP. A microprocessor translates differences in light phase and intensity into pressure measurement. Several companies have stopped producing these devices in recent years due to their performance and MR incompatibility, but one is still available − the MR Unsafe Camino® ICP Monitoring System (Natus).
MRI Safety Considerations
Except for fiberoptic transducer systems, nearly all ICP monitoring catheters available today are MR Conditional at 1.5T and 3.0T. Specific conditions vary by brand and model, but the following generic conditions generally apply.
- Verify that the ICP sensor is working properly prior to MRI. Disconnect all external cables, wires, and monitors.
- To prevent excessive RF-heating of the sensor tip, the non-removable wires and wire-containing tubing must not lie in a straight-line but be specially positioned in a coil-like configuration of several small loops on or near the patient's head. Sophysa even provides a special holder to wrap and secure the wires of their ICP sensor.
- Follow all manufacturer-mandated MRI technical parameter limits, including field strength, maximum spatial gradient field, slew rate, and SAR.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Nag, DS, Sahy S, Swain A, Kant S. Intracranial pressure monitoring: gold standard and recent innovations. World J Clin Cases 2019; 7:1535-1553. [DOI LINK]
Raboel PH, Bartek J, Andresen M, et al. Intracranial pressure monitoring: invasive versus non-invasive methods − a review. Crit Care Res Pract 2012; 950393. [DOI LINK]
Nag, DS, Sahy S, Swain A, Kant S. Intracranial pressure monitoring: gold standard and recent innovations. World J Clin Cases 2019; 7:1535-1553. [DOI LINK]
Raboel PH, Bartek J, Andresen M, et al. Intracranial pressure monitoring: invasive versus non-invasive methods − a review. Crit Care Res Pract 2012; 950393. [DOI LINK]
Related Questions
What safety precautions must be taken if a patient has a cerebrospinal fluid shunt?
What safety precautions must be taken if a patient has a cerebrospinal fluid shunt?