|
The chronic phase of a hematoma is arbitrarily defined to begin at about 2 months after the initial hemorrhage. By this time the original blood-containing cavity has largely collapsed, surrounding reactive edema has disappeared, RBCs have completely lysed, hemoglobin species have undergone degradation, and heme iron has been released and deposited in the surrounding tissues. The center and periphery of chronic hematomas have different pathological and imaging characteristics and must therefore be considered separately.
Chronic Hematoma Center
|
The residual central cavity of a chronic intracranial hematoma is filled with dark brown liquid and/or gelatinous material consisting primarily of water, with some dissolved proteins, fibrin, and hemoglobin degradation products. Some paramagnetic methemoglobin may still remain, but by 2 months most has been converted to hemichromes or even smaller protein fragments. Hemichromes, described in more detail in the next Q&A, are oxidatively denatured forms of met-Hb that cannot bind oxygen and have lost all paramagnetic properties. Very old hematomas may be slit-like without any internal structure and contain only clear or straw-colored fluid.
The imaging characteristics of the center of a chronic hematoma reflects its high water content. As such this region is typically hyperintense to brain on T2-weighted images and hypointense on T1-weighted images. Occasionally the center of older hematomas (especially subdurals) will remain bright for several months on T1-weighted images. This is most likely due to residual methemoglobin with possible contributions from soluble ferrous salts.
Diffusion characteristics also reflect high free water content, with only minimally restricted diffusion noted that depends on the protein content of the fluid. Thus the center of old hematomas will be dark on DWI/Trace images and bright on ADC maps.
Diffusion characteristics also reflect high free water content, with only minimally restricted diffusion noted that depends on the protein content of the fluid. Thus the center of old hematomas will be dark on DWI/Trace images and bright on ADC maps.
Chronic Hematoma Periphery
Around the periphery of older hematoma cavities, iron atoms released from degraded hemoglobin coalesce by the thousands into large, chunky metalloprotein complexes known as ferritin and hemosiderin. Ferritin and hemosiderin are engulfed by invading macrophages and ultimately deposit along the walls of old hematoma cavities. Outside the central nervous system they are also scavenged by reticulo-endothelial system (RES) cells of liver, lymph nodes, and bone marrow.
|
Ferritin and hemosiderin are superparamagnetic, with creating large microscopic susceptibility gradients in the areas where they accumulate. As water molecules diffuse through these gradients their resonance frequencies change with resultant T2/T2* dephasing.
The iron centers of ferritin and hemosiderin are sequestered and do not allow close approach of water for inner sphere relaxation. Hence only a minimal amount of T1 shortening occurs. |
The imaging characteristics of chronic hematomas are described in the table below, followed by a clinical example.
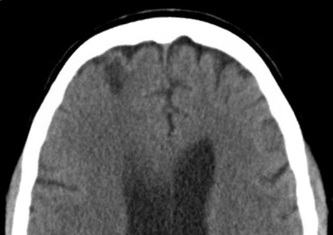
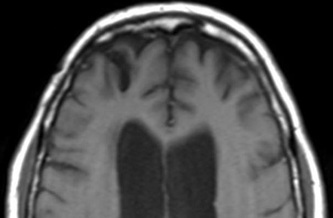
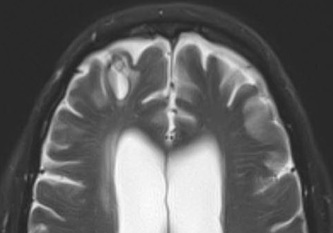
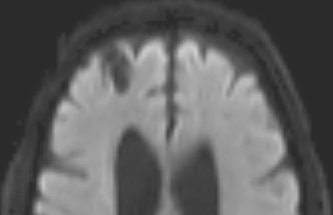
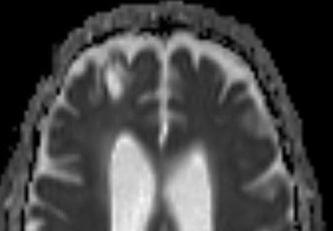
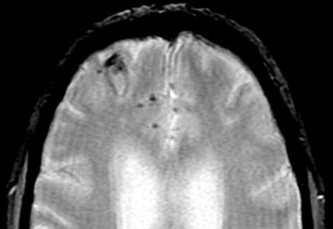
Above: Right frontal lobe hematoma in chronic phase (6 months) reflects high water content centrally as reflected by low CT attenuation (top left). Center of old hematoma is hypointense to brain on T1-weighted image (top center), hyperintense on T2-weighted image (top right), hypointense on DW Trace image (bottom left) and hyperintense on ADC map (bottom center). Marked hypointensity at periphery of hematoma noted on T2*-weighted gradient echo image (bottom right) due to ferritin/hemosiderin accumulation.
Advanced Discussion (show/hide)»
The peripheral microstructure of chronic hematomas depends on their anatomic location and thus may vary across the body. For example, chronic subdural hematomas develop an organized fibrovascular neomembrane attaching to the inner surface of the dura and surrounding the clot. Multiple membranes may develop formed by proliferating fibroblasts and thin-walled capillaries at risk for rebleeding. The walls of chronic subdural hematomas may thus have a thick, lamellated appearance.
References
Allkemper T, Tombach B, Schwindt W, et al. Acute and subacute intracerebral hemorrhages: comparison of MR imaging at 1.5 and 3.0 T–initial experience. Radiology 2004; 232:874–81.
Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology 1993; 189:15-26.
Gomori JM, Grossman RI. Mechanisms responsible for the MR appearance and evolution of intracranial hemorrhage. Radiographics 1988; 8:427-440.
Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cerebral Blood Flow Metabolism 2003; 23:629-652.
Allkemper T, Tombach B, Schwindt W, et al. Acute and subacute intracerebral hemorrhages: comparison of MR imaging at 1.5 and 3.0 T–initial experience. Radiology 2004; 232:874–81.
Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology 1993; 189:15-26.
Gomori JM, Grossman RI. Mechanisms responsible for the MR appearance and evolution of intracranial hemorrhage. Radiographics 1988; 8:427-440.
Wagner KR, Sharp FR, Ardizzone TD, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cerebral Blood Flow Metabolism 2003; 23:629-652.
Related Questions
What is ferritin? How is it different from hemosiderin?
What happens after the met-Hb stage and how do these later degradation products affect the MR signal?
What is ferritin? How is it different from hemosiderin?
What happens after the met-Hb stage and how do these later degradation products affect the MR signal?