Correct. All currently produced models are considered MR Unsafe.
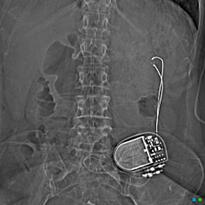
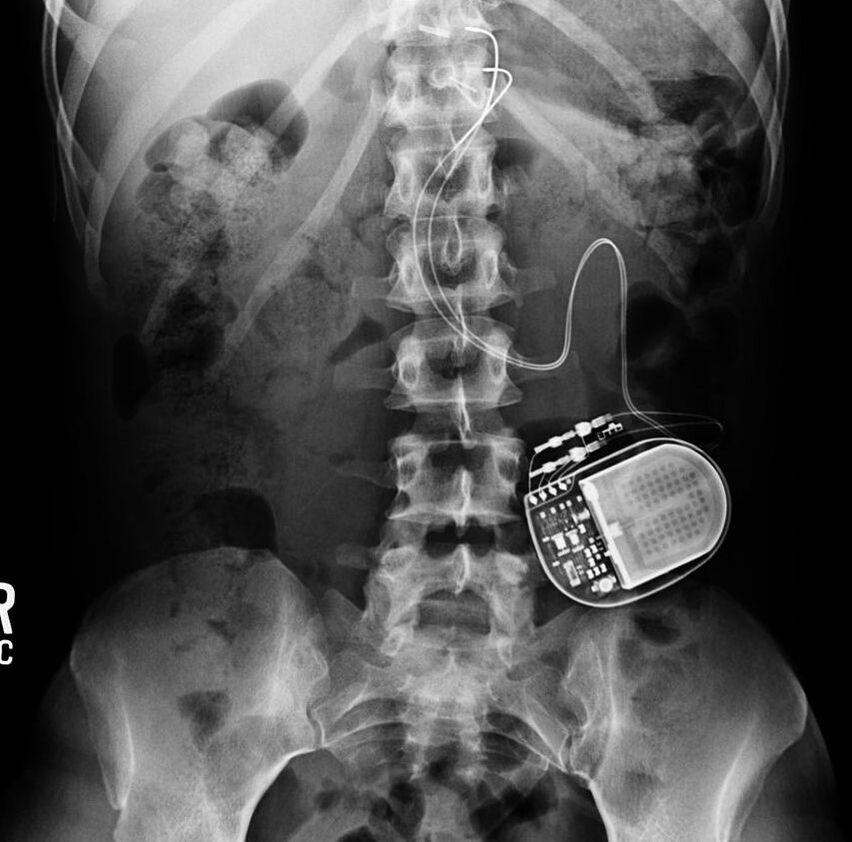
Gastric Electrical Stimulators (GES), sometimes called "gastric pacemakers', are designed to treat gastroparesis and obesity. Like cardiac pacemakers, gastric stimulators consist of a subcutaneously implanted pulse generator with leads that deliver electrical impulses. Three FDA and/or CE-approved devices are in use today: the Enterra® Therapy System (formerly known as the Transcend®), acquired by Medtronic; the VBLOC Maestro® Rechargeable System (EnteroMedics); and the abiliti® Closed Loop System (IntraPace).
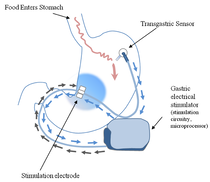
The electrodes of the Enterra® system are embedded in the stomach musculature, while those of the Maestro® are placed at the gastroesophageal junction next to the anterior and posterior vagal nerves. The abiliti® (which has the CE Mark for use in the EU but is not FDA-approved) operates on a feedback mechanism. A transgastric sensor in the fundus detects food intake, while gastric emptying is modified by electrical stimuli applied to the anterior vagus nerve along the lesser curvature of the stomach.
Two additional gastric stimulation systems have been marketed over the last decade but are no longer in production. These unremoved stimulators may potentially still be encountered in clinical practice so some familiarity with them as legacy devices is needed.
- The DIAMOND™ GES by Tantalus used three pairs of intramuscular electrodes implanted into the fundal, anterior, and posterior antral regions of the stomach. The device modulated gastric contraction, detecting the presence of food by changes of electrode impedence. Withdrawn from the market in 2017, it is considered MR Unsafe.
- The EndoStim® Lower Esophageal Sphincter Stimulation System was designed to treat gastrointestinal reflux disease by delivering electrical pulses to bipolar electrodes placed along the lower esophageal sphincter. Withdrawn from the market in 2018, retained EndoStim® devices are MR Conditional (with some very strict limitations).
Because of the worldwide obesity "epidemic", expect to see more GES's as well as other bariatric devices in the future. I also suspect that some of these will be considered MR Conditional as well.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc 2017; 50:11-16. [DOI LINK]
Horbach T, Thalheimer A, Seyfried F, et al. ability® Closed-Loop Gastric Electrical Stimulation System for treatment of obesity: clinical results with a 27-month follow-up. Obes Surg on line 2015. [DOI Link]
Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc 2017; 50:11-16. [DOI LINK]
Horbach T, Thalheimer A, Seyfried F, et al. ability® Closed-Loop Gastric Electrical Stimulation System for treatment of obesity: clinical results with a 27-month follow-up. Obes Surg on line 2015. [DOI Link]
Related Questions
Can I scan a patient with a retained PillCam™?
Can I scan a patient with a retained PillCam™?