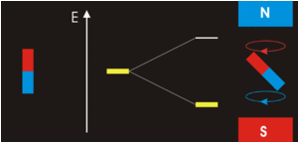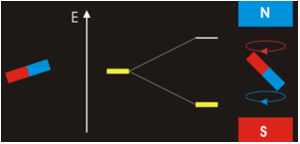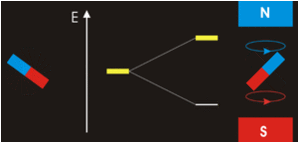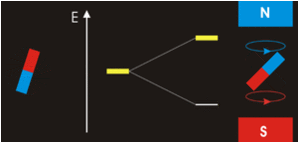
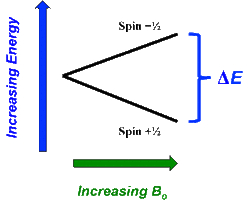
In the previous Q&A we introduced the concept of the two nuclear spin states of the ¹H nucleus, commonly denoted as |+½⟩ and |-½⟩. We also showed how the separation and measurement of such states could be obtained by passing the spins through a magnetic field (Bo) as Stern and Gerlach did in 1922. Because the spins tend to sort themselves into orientations either generally aligned with or opposed to the external field, the two spin states are often referred to as "spin-up" or "parallel" and "spin-down" or "anti-parallel".
The physical separation of spin-up and spin-down particles in the Stern-Gerlach apparatus reflects an energy difference (ΔΕ) between the two states. This is known as the nuclear Zeeman effect, named after Pieter Zeeman, who in 1896 had observed the splitting of optical spectral lines by a magnetic field.
Our experience in the macroscopic world informs us that physical energy is required to to push the like poles of two bar magnets close together. Their natural (and lower energy) state is to be in parallel, not in opposition. By analogy, the lower energy state for a nuclear spin in an external field is spin-up/parallel, while the higher energy state is spin-down/anti-parallel.
Warning: Although we have described two pure states for the nucleus (spin-up and spin-down), it is incorrect to infer that spins physically reside only in one of these two states or that they "flip" from one state to another. See the next Q&A for a more complete discussion.
In the subatomic world governed by quantum mechanics, not only spin angular momentum but also the transfer of energy may assume only discrete units. In 1905 Max Planck showed the relationship between the change in energy (ΔE) of an atomic system by emission of a photon of frequency (fo) to be:
ΔE = h fo
where h is Planck's constant whose value is approximately 6.626 x 10-34 Joule-sec. Planck's constant reflects the granularity of the subatomic world and the fact that energy is released or absorbed only in discrete packets or quanta.
|
The energy gap (ΔΕ) between two nuclear spin states scales directly with magnetic field strength and is given by the Zeeman equation:
ΔΕ = γ h Bo
where γ is called the gyromagnetic ratio, a constant specific to a particular nucleus. For the ¹H nucleus, the value of γ is 42.58 MHz/Tesla.
The Planck relation and Zeeman equation may be combined, producing an interesting and important result that will look familiar to those with prior exposure to MRI: |
E = h fo = γ h Bo
or
fo = γ Bo
or
fo = γ Bo
This is the famous Larmor equation, showing that NMR resonance frequency (fo) is simply the gyromagnetic ratio (γ) times the magnetic field strength (Bo). Of course, this is not a rigorous derivation from quantum mechanics, but does show how directly the Larmor relation results from very basic concepts that should be understandable to most readers. The fact that Planck's constant (h) disappears from the solution implies that a non-quantum explanation using classical physics is also possible.
If you don't understand everything right now, don't worry. We will have much, more more to say about the Larmor equation in later Q&A's!
If you don't understand everything right now, don't worry. We will have much, more more to say about the Larmor equation in later Q&A's!
Advanced Discussion (show/hide)»
Because the electron has a negative gyromagnetic ratio (γ), its magnetic moment points opposite to the direction of its spin. The spin-up state |+½> is therefore the higher energy level. Also, the direction of electron precession with respect to Bo is actually opposite that of the hydrogen proton,
References
"Nuclear Magnetic Resonance." Wikipedia, The Free Encyclopedia.
Cresser JD. Particle spin and the Stern-Gerlach experiment. Lecture notes in quantum mechanics, Chapter 6, from: physics.mq.edu.au/~jcresser/Phys301.html (27 April 2009)
"Nuclear Magnetic Resonance." Wikipedia, The Free Encyclopedia.
Cresser JD. Particle spin and the Stern-Gerlach experiment. Lecture notes in quantum mechanics, Chapter 6, from: physics.mq.edu.au/~jcresser/Phys301.html (27 April 2009)
Related Questions
What is spin?
How do you predict the value of nuclear spin (I) based on the number of protons and neutrons?
Where does the energy come from to keep the precession going?
What is spin?
How do you predict the value of nuclear spin (I) based on the number of protons and neutrons?
Where does the energy come from to keep the precession going?