Not necessarily. An analogy is often made to the well-recognized superiority of nonionic iodine-based contrast agents over ionic ones in terms of renal safety and adverse reactions. The (false) conclusion is that non-ionic gadolinium agents would likewise be better than ionic ones.

Ionicity is closely related to osmolality (the number of dissolved particles per kg of water). A comparison of the gadolinium agents in clinical use are shown in the table right. As a point of reference, normal serum osmolality is approximately 290 mOsm/kg, so even non-ionic contrast agents are still significantly hypertonic with respect to blood.
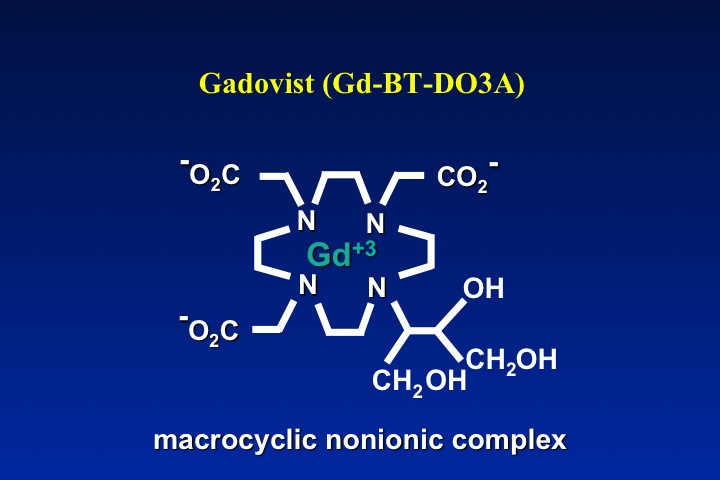
A close tracking between ionicity and high osmolality is noted. The only exception on the table is Gadavist®, which although non-ionic has high osmolality. This is the result of the manufacturer's decision to dispense this drug in a concentration twice as high as the other agents.
A close tracking between ionicity and high osmolality is noted. The only exception on the table is Gadavist®, which although non-ionic has high osmolality. This is the result of the manufacturer's decision to dispense this drug in a concentration twice as high as the other agents.
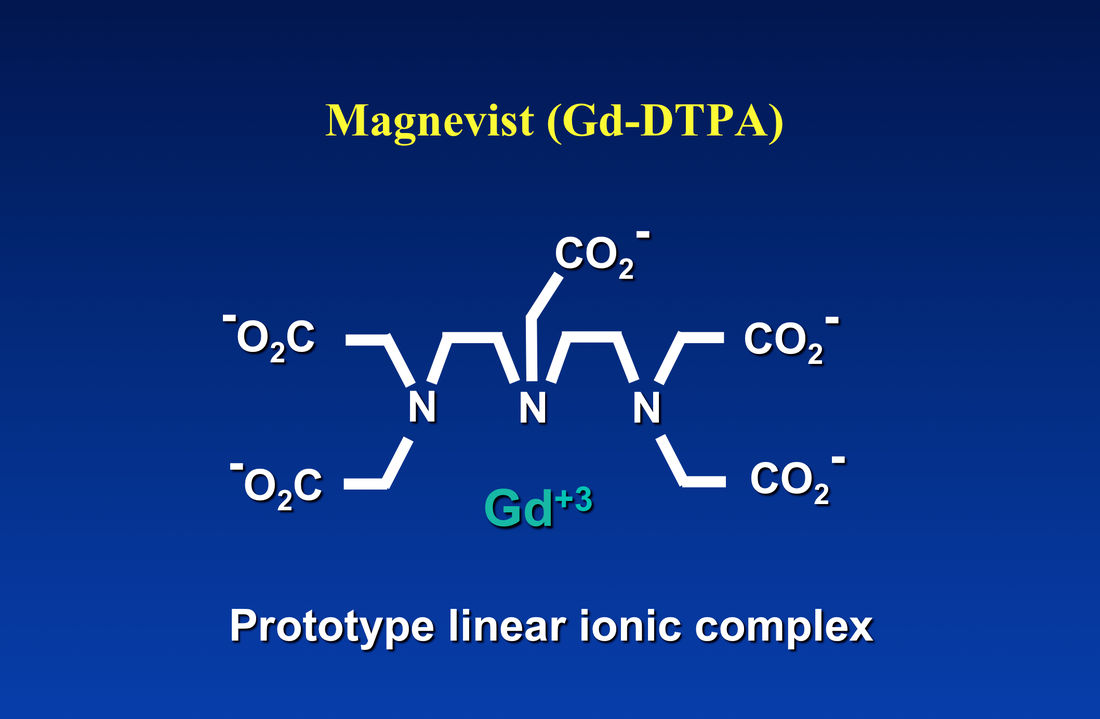
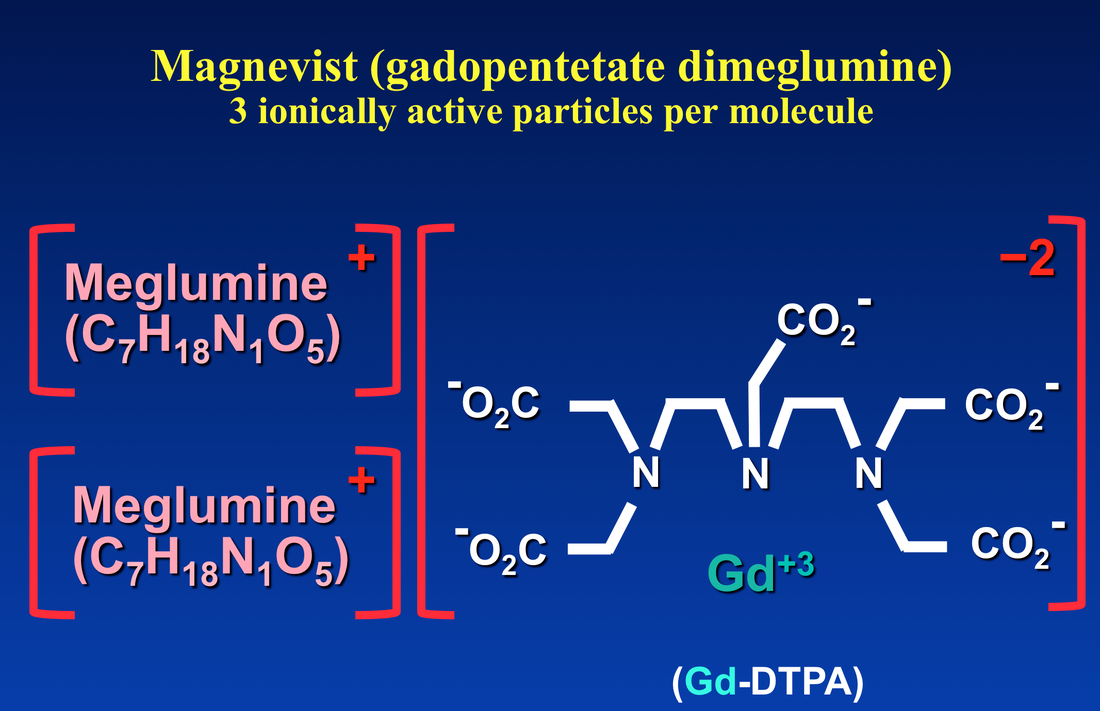
The net charge of the gadolinium ion plus its ligand controls the compound's osmolality. As an example, let us consider the venerable Gd-DTPA anionic portion of Magnevist® with net charge of −2. To attain electroneutrality, two meglumine cations (each with charge +1) are attached to the salt. When dissolved in solution three osmotically active particles are released, explaining why Magnevist® has an osmolality of roughly three times higher than a non-ionic agent like ProHance®.
|
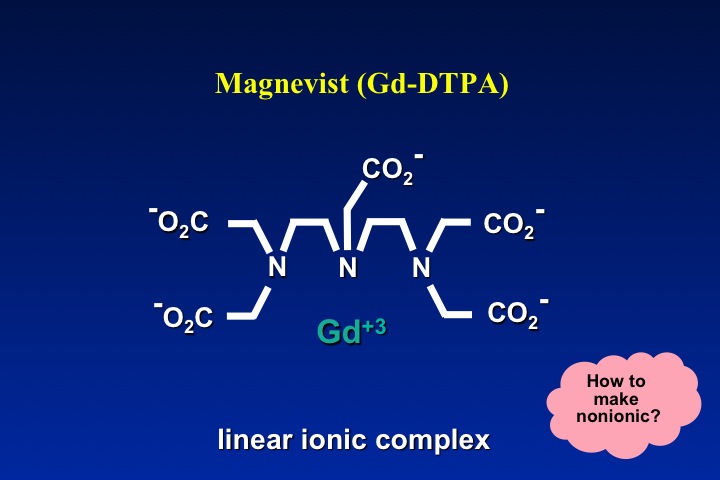
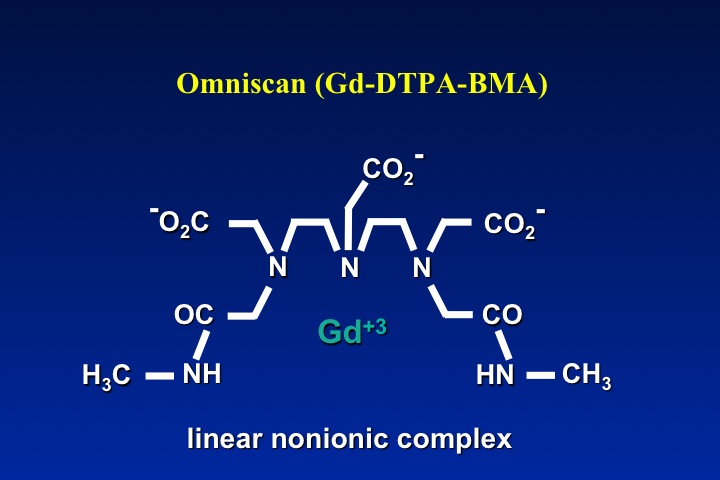
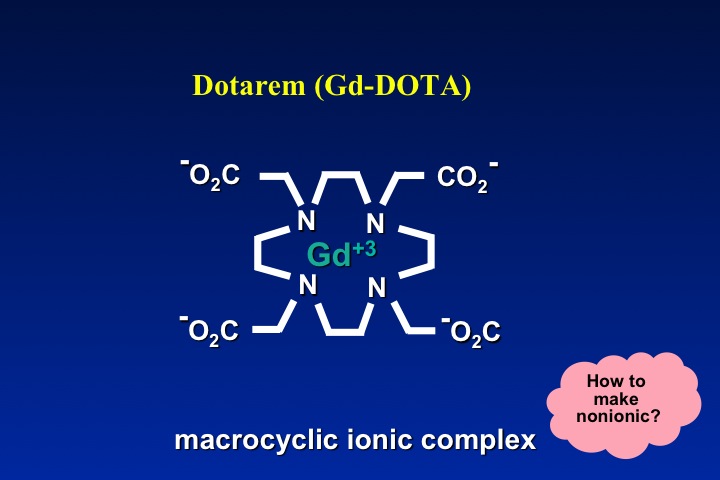
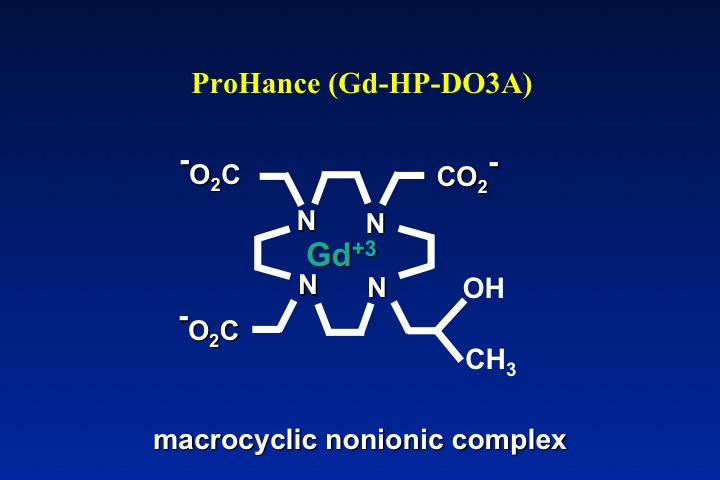
The obvious method to make a gadolinium contrast agent non-ionic is to attach a ligand whose charge is −3. This will balance out the +3 charge of the Gd ion and make the molecule electrically neutral. By toggling through the slide show left, you can see how manufacturers have modified various ligand to achieve electroneutrality for several commercially used contrast agents. The same process can be applied to both linear and macrocyclic agents.
|
So, does ionicity/osmolality matter in selection of a Gd-contrast agent?
Three clinical scenarios must be considered where a difference might be important: 1) subcutaneous extravasation of contrast; 2) potential nephrotoxicity; and 3) problems related to acute increase in serum osmolality.
Three clinical scenarios must be considered where a difference might be important: 1) subcutaneous extravasation of contrast; 2) potential nephrotoxicity; and 3) problems related to acute increase in serum osmolality.
Tissue irritation from extravasation is somewhat more severe when hyperosmolar contrast is used. Because gadolinium agents are injected in such low volumes (10-15 cc), however, even large fraction extravasations of either type of agent do not generally create significant injury. In my opinion, the slightly better performance of low osmolar/non-ionic agents in this dimension should not outweigh other factors in selection of a contrast material.
A similar conclusion can be drawn concerning possible acute renal toxicity. By the time contrast has circulated through the heart and aorta, its concentration has been significantly diluted. Thus, whatever mild nephrotoxic effects gadolinium contrast may possess (see separate Q&A), the difference between ionic and nonionic formulations is probably minimal.
Systemic osmolar effects of gadolinium contrast are not likely to be significant for a similar reason. The osmolar effect of a contrast agent is determined not by its concentration in the bottle, but by its concentration in the blood. The systemic concentration of a contrast agent depends on its total osmotic load, which is the drug's pharmaceutical osmolality times the volume administered.
For example, the total osmolar load from a 15-cc dose of Magnevist® is (1940) x (0.015), or 29 mOsm, whereas the osmolar load from the same dose of ProHance® would be (630) x (0.015), or 9 mOsm. Administration of these drugs would increase serum osmolality by only 1.5% and 0.5% respectively. Iodinated agents, however, are administered in much larger volumes, so that their systemic osmolar effects are much greater. For example, the total osmolar load from 150 cc of Renografin-60® (osmolality, 1420 mOsm/L) is calculated to be 213 mOsm, whereas the osmolar load from 150 cc of the non-ionic agent Isovue-300 (osmolality, 616 mOsm/L) is 92 mOsm. Serum osmolality would potentially increase by 12% and 5% respectively from administration of these iodine-based agents.
This quantitative example demonstrates that even the most ionic gadolinium agents produce a trivial change in serum osmolality, an effect far less than even a non-ionic iodine agent. The difference between ionic and nonionic gadolinium agents in terms of their systemic osmotic effects is therefore hardly noticeable.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systemic review and meta-analysis. Radiology 2018; 286:471-482. (Surprisingly, Omniscan had the lowest rate of reactions, and macrocyclics had higher rates than linear agents)
Bellin M-F, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol 2008; 66:160-167.
Laurent S, Vander Elst L, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging 2006;1:128-137.
Rohrer M, Bauer H, Mintorovitch J et al. Comparison of magnetic properties of MRI contrast media solutions a different magnetic field strengths. Invest Radiol 2005; 40:715-724.
Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: a systemic review and meta-analysis. Radiology 2018; 286:471-482. (Surprisingly, Omniscan had the lowest rate of reactions, and macrocyclics had higher rates than linear agents)
Bellin M-F, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol 2008; 66:160-167.
Laurent S, Vander Elst L, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging 2006;1:128-137.
Rohrer M, Bauer H, Mintorovitch J et al. Comparison of magnetic properties of MRI contrast media solutions a different magnetic field strengths. Invest Radiol 2005; 40:715-724.
Related Questions
So many gadolinium contrast agents are now available. What are the differences among them?
So many gadolinium contrast agents are now available. What are the differences among them?