
No. The risk of NSF varies according to the type of contrast agent used, divided into 3 groups by the American College of Radiology (ACR).
Group I agents are historically associated with the highest risk of NSF. The vast majority of NSF cases have been reported with Omniscan® (gadodiamide), while the second highest number have been reported with Magnevist® (gadopentetate). Optimark™ (gadoversetamide) holds the non-enviable third position, a surprising result due to its relatively low market penetrance and use. These agents, all with a linear structure, have been banned for sale in most of Europe, and are prohibited from being advertised in the United States.
Group I agents are historically associated with the highest risk of NSF. The vast majority of NSF cases have been reported with Omniscan® (gadodiamide), while the second highest number have been reported with Magnevist® (gadopentetate). Optimark™ (gadoversetamide) holds the non-enviable third position, a surprising result due to its relatively low market penetrance and use. These agents, all with a linear structure, have been banned for sale in most of Europe, and are prohibited from being advertised in the United States.
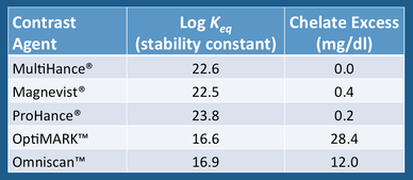
The high prevalence of NSF cases associated with Ominscan™ and Optimark™ is likely related to the fact that these drugs have relatively weak binding of gadolinium to the chelate. This is reflected in the values of their thermodynamic stability constants, shown in the table right. In that our current concepts of NSF pathophysiology relate to deposition of free Gd in tissues, it makes sense that contrast agents more likely to dissociate would carry a higher risk of the disease.
The relatively high numbers of NSF cases associated with Magnevist® is probably not explainable by weak ligand binding, since its dissociation constant is five orders of magnitude higher than Ominscan™ and Optimark™. One explanation is that Magnevist® may be overrepresented in the total number of reported NSF cases simply because of its dominant market share and high worldwide use.
Cases of NSF have have rarely been reported with Group II agents, and nearly all of these are considered "confounded" — meaning that these patients received two or more different gadolinium compounds and hence causation by a single agent could not be established. However, (non-confounded) single agent cases of NSF have been reported for Gadavist®, MultiHance®, Dotarem®, and ProHance®.
To my knowledge no cases of NSF have been reported with the relatively new gadolinium agent, Eovist® (gadoxetate) or the very new Vueway®/Elucerim® (gadopiclenol). Because of limited data and worldwide experience, these agents were initially placed by the ACR in a separate category (Group III), although gadopiclenol was moved to Group II in 2023. Eovist® has been around long enough that it could be justifiably moved up to Group II in my opinion. (This is the recommendation of the Canadian Association of Radiologists, but not the ACR).
Notwithstanding the ACR Classification model, US Food and Drug Administration requires the following black box warning to be included in product literature for all gadolinium-based contrast agents:
In conclusion, even though some contrast agents have not been directly implicated in the development of NSF, no gadolinium-based contrast formulation should be considered completely free of risk. Based on the available data, we avoid the use of all ACR Group I agents in all patients.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
ACR Committee on Drugs and Contrast Agents. ACR Manual on Contrast Agents, 2024. American College of Radiology, 2024.
Attari H, Cao Y, Elmholdt TR, et al. A systematic review of 639 patients with biopsy-confirmed nephrogenic system fibrosis. Radiology 2019; 292:376-386.
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus 2010; 3:285-287. [DOI LINK]
Endrikat J, Dohanish S, Schleyer N, et al. 10 Years of Nephrogenic Systemic Fibrosis: A comprehensive analysis of nephrogenic systemic fibrosis reports received by a pharmaceutical company form 2006 to 2016. Invest Radiol 2018; 53:541-550. [DOI LINK] (article sponsored by Bayer, so much be viewed with a critical eye, but makes convincing case that Magnevist® was statistically overrepresented in the percentage of NSF cases due to its decades of use and dominant market share).
Lim YU, Bang J, Ko Y, et al. Late onset nephrogenic systemic fibrosis in a patient with Stage 3 chronic kidney disease: a case report. J Korean Med Sci 2020; 35:e293. [DOI LINK] (perhaps the most recent documented case of NSF, a man who developed the disease in 2019 after receiving a single dose of Dotarem®).
Lohani S, Golenbiewski J, Swami A, Halalau A. A unique case of nephrogenic systemic fibrosis from gadolinium exposure in a patient with normal eGFR. BMJ Case Rep 2017; Oct11;2017:bcr2017221016 [DOI LINK] (Case of NSF due to MultiHance® alone; Although patient had normal eGFR, she was extremely thin with poor muscle mass and low serum creatinine levels that probably led to overestimation of her GFR).
Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol 2008; 66:175-179.
Rofsky NM, Sherry AD, Lenkinski RE. Nephrogenic systemic fibrosis: a chemical perspective. Radiology 2008; 247:608-612.
Schema N, Maralani PJ, Hurrell C, et al. Updated clinical practice guideline on the use of gadolinium-based contrast agents in kidney disease issued by the Canadian Association of Radiologists. Can Assoc Radiol J 2019; 70:226-232. [DOI LINK]
Starekova J, Bruce RJ, Sadowski EA, Reeder SB. No cases of nephrogenic system fibrosis after administration of gadoxetic acid. Radiology 2020; 297;556-562. [DOI LINK]
US Food and Drug Administration. FDA drug safety communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm (updated 12/2010)
Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus statements fro the American College of Radiology and the National Kidney Foundation. Radiology 2021; 298:28-35. [DOI LINK]
Woolen SA, Shankar PR, Gagnier JJ, et al. Risk of nephrogenic systemic fibrosis in patients with Stage 4 or 5 chronic kidney disease receiving a Group II gadolinium-based contrast agent. A systemic review and meta-analysis. JAMA Intern Med 2020; 180:223-230. [DOI LINK] (Risk < 0.07%)
Young LK, Matthew SZ, Houston JG. Absence of potential gadolinium toxicity symptoms following 22,897 gadoteric acid (Dotarem®) examinations, including 3,209 performed on renally insufficient individuals. Eur Radiol 2019; 29:1922-30. (but see cases reported by Endrikat and Lim).
ACR Committee on Drugs and Contrast Agents. ACR Manual on Contrast Agents, 2024. American College of Radiology, 2024.
Attari H, Cao Y, Elmholdt TR, et al. A systematic review of 639 patients with biopsy-confirmed nephrogenic system fibrosis. Radiology 2019; 292:376-386.
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus 2010; 3:285-287. [DOI LINK]
Endrikat J, Dohanish S, Schleyer N, et al. 10 Years of Nephrogenic Systemic Fibrosis: A comprehensive analysis of nephrogenic systemic fibrosis reports received by a pharmaceutical company form 2006 to 2016. Invest Radiol 2018; 53:541-550. [DOI LINK] (article sponsored by Bayer, so much be viewed with a critical eye, but makes convincing case that Magnevist® was statistically overrepresented in the percentage of NSF cases due to its decades of use and dominant market share).
Lim YU, Bang J, Ko Y, et al. Late onset nephrogenic systemic fibrosis in a patient with Stage 3 chronic kidney disease: a case report. J Korean Med Sci 2020; 35:e293. [DOI LINK] (perhaps the most recent documented case of NSF, a man who developed the disease in 2019 after receiving a single dose of Dotarem®).
Lohani S, Golenbiewski J, Swami A, Halalau A. A unique case of nephrogenic systemic fibrosis from gadolinium exposure in a patient with normal eGFR. BMJ Case Rep 2017; Oct11;2017:bcr2017221016 [DOI LINK] (Case of NSF due to MultiHance® alone; Although patient had normal eGFR, she was extremely thin with poor muscle mass and low serum creatinine levels that probably led to overestimation of her GFR).
Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol 2008; 66:175-179.
Rofsky NM, Sherry AD, Lenkinski RE. Nephrogenic systemic fibrosis: a chemical perspective. Radiology 2008; 247:608-612.
Schema N, Maralani PJ, Hurrell C, et al. Updated clinical practice guideline on the use of gadolinium-based contrast agents in kidney disease issued by the Canadian Association of Radiologists. Can Assoc Radiol J 2019; 70:226-232. [DOI LINK]
Starekova J, Bruce RJ, Sadowski EA, Reeder SB. No cases of nephrogenic system fibrosis after administration of gadoxetic acid. Radiology 2020; 297;556-562. [DOI LINK]
US Food and Drug Administration. FDA drug safety communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm (updated 12/2010)
Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus statements fro the American College of Radiology and the National Kidney Foundation. Radiology 2021; 298:28-35. [DOI LINK]
Woolen SA, Shankar PR, Gagnier JJ, et al. Risk of nephrogenic systemic fibrosis in patients with Stage 4 or 5 chronic kidney disease receiving a Group II gadolinium-based contrast agent. A systemic review and meta-analysis. JAMA Intern Med 2020; 180:223-230. [DOI LINK] (Risk < 0.07%)
Young LK, Matthew SZ, Houston JG. Absence of potential gadolinium toxicity symptoms following 22,897 gadoteric acid (Dotarem®) examinations, including 3,209 performed on renally insufficient individuals. Eur Radiol 2019; 29:1922-30. (but see cases reported by Endrikat and Lim).

