|
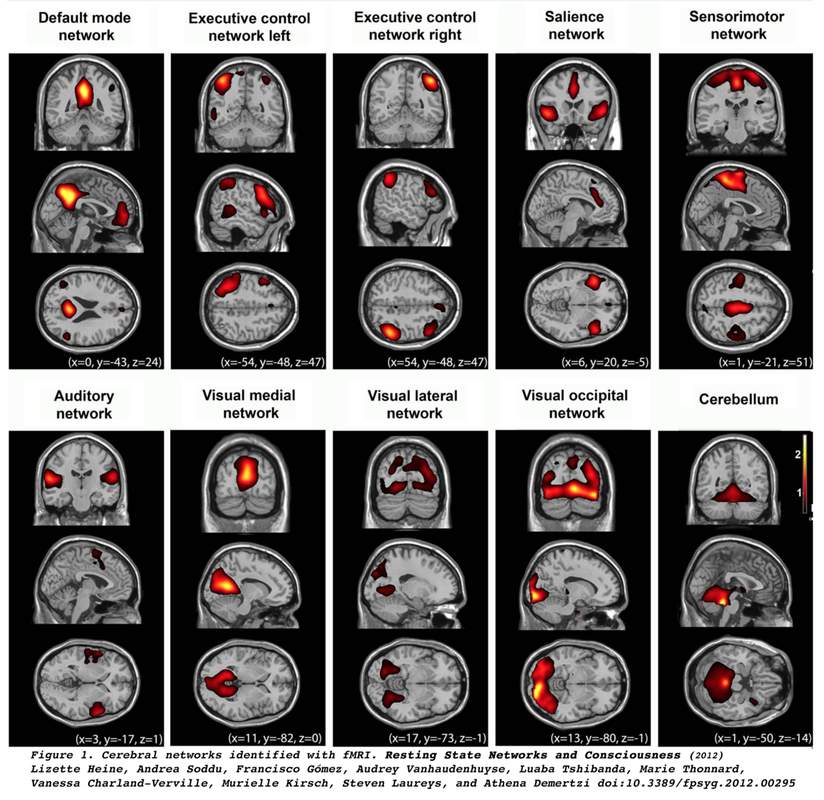
Resting-state fMRI (RS-fMRI) traces its origin to the work of Biswal et al (1995) who demonstrated highly correlated low frequency (<0.1 Hz) changes in BOLD signal between sensorimotor and supplementary motor cortices bilaterally in subjects at rest. They also noted similar synchronous fluctuations in the auditory and visual systems, recognizing all of these to be manifestations of the brain's functional connectivity.
|
Spontaneous fluctuation in BOLD signal during RS-fMRI
|
|
Short video overview of RS-fMRI
|
RS-fMRI is similar to conventional fMRI, but does not require subjects to perform a task or respond to stimuli. Subjects merely lie in the scanner for 5-10 minutes with their eyes closed or staring at a fixed point while whole-brain BOLD data is collected. Typically T2*-weighted echo-planar images are obtained with 3-4 mm isotropic spatial resolution, TR values of 2-3 sec, and multi-band acceleration (if available). A benefit of its task-free nature is that RS-fMRI can be performed on all types of subjects, including infants, children, patients with neurological disabilities, patients under anesthesia, and even animals.
|
Advanced Discussion (show/hide)»
Further Comments on Reseting State Networks (RSN's)
Of all the RSN’s the default mode network (DMN) has perhaps garnered the most interest, as it is thought to be involved in introspection and episodic memory. Anatomically the DMN includes the precunei, posterior cingulate gyri, inferior-medial frontal lobes, and lateral parietal lobes. The defining characteristic of the DMN is that it is most active during rest and deactivates during performance of tasks demanding attention.
Interestingly, many of the RSN’s detected in humans are present in monkeys and lower mammals. Identification of these networks has shed light on the functioning of the brain in normal and pathologic states. Considerable accumulated research now shows disruption or changes in RSN’s by diseases such as dementia, psychiatric disorders, substance abuse, head trauma, migraine, and sleep disturbances. See the review articles below for an introduction.
Further Comments on Technical Details of RS-fMRI
No uniform consensus exists concerning whether RS-fMRI studies should be performed eyes closed, eyes open staring at a blank screen, or eyes focused on a dot or cross. If the eyes shut method is chosen, it should be realized that a substantial fraction of subjects will fall asleep after 5-10 minutes which may alter the results. We prefer an eyes open technique for this reason. Whatever method is chosen, it is important to apply this consistently for all subjects in an experimental setup.
Similarly the effects of recent food, caffeine, nicotine, or alcohol shortly before the RS-fMRI study should be controlled/standardized, as these can have effects on the BOLD signal and correlation values.
Because respiratory and cardiac cycles contain low-frequency oscillations close to those in RS-fMRI, it is often advisable to simultaneously monitor pulse and respiration during the scan as well.
Typical imaging parameters at 3T for RS-fMRI studies include: TR = 2000-3000 ms, TE= 30-40 ms, α = 80-90°, and voxel size = 3-4 mm. If multiband is available the study may be shortened or used to obtain better spatial or temporal resolution. Typical values in this scenario include: TR = 500-1500 ms, TE= 30-40 ms, α = 50-70°, and voxel size = 2-3 mm.
Although RS-fMRI studies are by definition “task free”, it is possible to alter the sensory conditions between runs to determine how connectivity patterns might change. For example, a subject could have three 10-minute RS-fMRI sessions performed back to back: 1) a control session staring at a cross on a white screen; 2) a “happy” session viewing bunnies, puppies, and ducks; 3) a “scary” session viewing frightening or emotionally disturbing images.
Likewise, subjects may be studied sequentially over time before and after undergoing chemotherapy, surgery, or some type of intervention. RS-fMRI may also be used in conjunction with task-based experiments, where resting-state fluctuations are “subtracted” from the task-based data, producing less noisy activation maps.
Arabshahi S, Chung S, Alivar A, et al. A comprehensive and broad approach to resting-state functional connectivity in adult patients with mild traumatic brain injury. AJNR Am J Neuroradiol 2024; 45:637-646. [DOI LINK]
Barkhof F, Haller S, Rombout SARB. Resting-state functional MR imaging: a new window to the brain. Radiology 2014; 272:29-49. [DOI Link] (good recent review)
Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34:537–41. [DOI Link] (first description of across-brain correlations of resting fMRI signals)
Christoff K, Gordon AM, Smallwood J, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. PNAS 2009; 106:8719-8724. [DOI Link]
Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci (USA) 2003;100:253–58. [DOI Link] (first identification of DMN using fMRI)
Lv H, Wang Z, Tong LM, et al. Resting-state functional MRI: Everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol 2018; 39:1390-99. [DOI LINK]
Patriat R, Molloy EK, Meier TB, et al. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. NeuroImage 2013;78:463–473. [DOI Link]
Power JD, Schlaggar BL, Petersen SE. Studying brain organization via spontaneous fMRI signal. Neuron 2014; 84:681-696. [DOI Link]
Rack-Gomer AL, Liu TT. Caffeine increases the temporal variability of resting-state BOLD connectivity in the motor cortex. NeuroImage 2012; 59:2994–3002. [DOI Link]
Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci (USA) 2001; 98:676-682. [DOI Link]
Seitzman BA, Snyder AZ, Leuthardt EC, Shimony JS. The state of resting state networks.Top Man Reson Imaging 2019; 28:189-196. [DOI Link] (excellent recent review).
Van Dijk et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010; 103:297-321. [DOI Link]
How do you process and analyze resting state fMRI data?