|
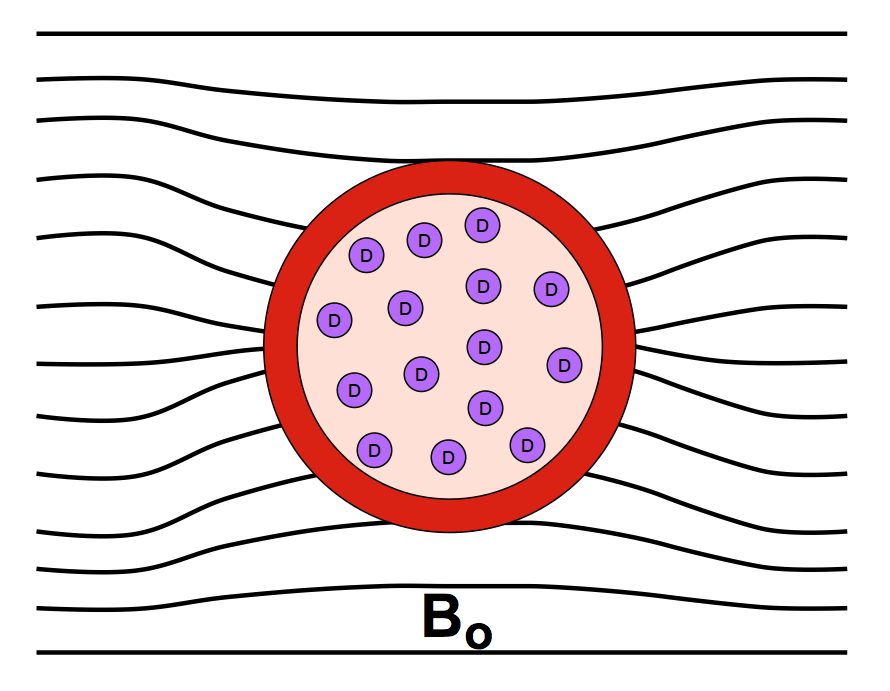
BOLD (Blood Oxygen Level Dependent) contrast results from changing regional blood concentrations of oxy- and deoxy-hemoglobin. As described in a prior Q&A, oxyhemoglobin has no unpaired electrons and is weakly diamagnetic. When oxygen is released to form deoxyhemoglobin, 4 unpaired electrons are exposed at each iron center, causing the molecule to become strongly paramagnetic. The BOLD effect is directly related to the concentration of deoxy-hemoglobin, which varies from less than 2% in arterial blood to greater than 40% in venous blood.
|
|
The presence of paramagnetic deoxyhemoglobin within red blood cells creates local magnetic field distortions (susceptibility gradients) in and around blood vessels. These local field disturbances cause nearby stationary and slowly moving spins to have different resonance frequencies and phase shifts. The resultant intravoxel dephasing is a classic T2*-shortening effect most prominent near larger veins and accentuated by use of GRE sequences with echo times (TEs) close to T2*. The effect scales linearly with field strength (Bo) and is the dominant mechanism for BOLD contrast at 1.5T.
|
Advanced Discussion (show/hide)»
At least four different mechanisms produce T2 and T2* relaxation in BOLD fMRI, more completely described in the references below. These include both intravascular and extravascular components, whose pools can be considered separately because the exchange rate between the compartments is slow compared to imaging time (TE). Diffusion effects are always important because during the typical time course of an fMRI experiment (TE ≈ 50 ms) a water molecule diffuses approximately 4 capillary diameters. The orientation of blood vessels relative to the direction of Bo must also be considered.
One of the major differences between BOLD image contrast mechanisms at lower (≤ 3.0T) versus higher (≥ 7.0T) fields results from field-related changes in relaxation times for blood and brain. At 1.5 the T2's of arterial and venous blood are about 250 and 180 msec respectively, while white matter and gray matter lie in the range of 70-100 msec. At 7.0T the relative relaxation times are reversed, with T2 (and T2*) of blood becoming extremely short compared to those of brain.
Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci 1936; 22:210-216. (first paper describing and explaining the diagmagnetic and paramagnetic properties of oxy- and deoxy-hemoglobin respectively)
Silvennoinen MJ, Clingman CS, Golay X, et al. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med 2003; 49:47–60.
Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochem Biophys Acta 1982; 714:265–270. (describes T2 changes due to diffusion and their quadratic dependence on field strength)
Uludağ K, Muller-Bierl B, Uğurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 2009; 48:150-165.
Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med 1994; 31:601-610.
What are the different forms of hemoglobin and why do they have different magnetic properties?
Why does acute hemorrhage become dark on T2-weighted images?
Why does the BOLD signal increase during activation? It seems like it should decrease since more oxygen is being used up.