|
Upper Airway Devices
All conventional temporary oral and nasopharyngeal airway tubes are made entirely of plastic or silicone and are considered MR Safe. Two specialized nasal airway devices contain metal and are MR Conditional:
|
(Endo)tracheal Tubes
"Conventional" endotracheal tubes (ETTs) are made almost entirely from plastic and can be considered safe for use in the MR environment. Those with inflatable cuffs, however, are technically classified as MR Conditional because they contain a tiny metal spring in the pilot balloon valve (where air for the cuff is injected). The valve should be taped down to the Y-connector of the ventilator circuit at least 3 cm away from any area of imaging interest (to avoid susceptibility artifact). The pilot valve springs of several newer tubes are non-ferromagnetic, reducing or eliminating such artifacts.
"Reinforced" ETTs contain a metal coil (usually stainless steel) spirally wound in the wall of the tube to prevent kinking. Most manufacturers have declared their models to be MR Unsafe but some brands are rated as MR Conditional. If using such a conditional ETT, the airway should be fixed properly in place with adhesive tape, cloth tape or other appropriate means to prevent movement or dislodgement before entering the MR environment. Expect a large artifact over the neck and upper chest from the reinforcing wire.
 Laryngeal Mask Airway (LMA)
Laryngeal Mask Airway (LMA)
Extraglottic Airway Devices
Extraglottic airway devices, more commonly known under their trade name, Laryngeal Mask Airways (LMAs), are an alternative to tracheal intubation, especially in children undergoing MRI. They are inserted orally after induction of general anesthesia and fit tightly over the top of the larynx and pyriform fossae. MR safety issues mirror those of ETTs, depending on their composition. Most LMAs are all-plastic with just a pilot valve spring, so are (trivially) MR Conditional while some (uncommon) wire-reinforced models are rated MR Unsafe.
Extraglottic airway devices, more commonly known under their trade name, Laryngeal Mask Airways (LMAs), are an alternative to tracheal intubation, especially in children undergoing MRI. They are inserted orally after induction of general anesthesia and fit tightly over the top of the larynx and pyriform fossae. MR safety issues mirror those of ETTs, depending on their composition. Most LMAs are all-plastic with just a pilot valve spring, so are (trivially) MR Conditional while some (uncommon) wire-reinforced models are rated MR Unsafe.
Because LMAs are much larger than ETTs, they significantly distend the hypopharynx when in place. As such, radiologists should be aware that LMAs produce significant anatomic distortions in this region and can compress or displace mass lesions.
 "MR Unsafe" Jackson stainless steel tracheotomy tube with obturator
"MR Unsafe" Jackson stainless steel tracheotomy tube with obturator
Larynx/Laryngectomy Implants and Devices
The MR safety of tracheostomy and laryngectomy tubes depends on their composition and design. Stainless steel tubes like that pictured right are, of course, contraindicated. All plastic tracheostomy tubes are MR Safe (unless they contain a spring-loaded pilot cuff valve, which should be taped away securely as with conventional cuffed ETTs). As with ETTs, the declared safety of wire-reinforced tracheotomy tubes is largely dependent on the risk-tolerance of the manufacturer. Some tubes are now reinforced with titanium rather than stainless steel resulting in considerably less susceptibility artifacts over the lower neck.
Laryngectomy accessories (used by patients with permanent stomas) include short, uncuffed LaryTubes™, base plates, buttons, studs, covers, and HME (Heat and Moisture Exchange) filters. Except for certain tracheostoma speech valves described below, essentially all wearable laryngectomy accessories are made of polyurethane or soft silicone and considered MR Safe.
The MR safety of tracheostomy and laryngectomy tubes depends on their composition and design. Stainless steel tubes like that pictured right are, of course, contraindicated. All plastic tracheostomy tubes are MR Safe (unless they contain a spring-loaded pilot cuff valve, which should be taped away securely as with conventional cuffed ETTs). As with ETTs, the declared safety of wire-reinforced tracheotomy tubes is largely dependent on the risk-tolerance of the manufacturer. Some tubes are now reinforced with titanium rather than stainless steel resulting in considerably less susceptibility artifacts over the lower neck.
Laryngectomy accessories (used by patients with permanent stomas) include short, uncuffed LaryTubes™, base plates, buttons, studs, covers, and HME (Heat and Moisture Exchange) filters. Except for certain tracheostoma speech valves described below, essentially all wearable laryngectomy accessories are made of polyurethane or soft silicone and considered MR Safe.
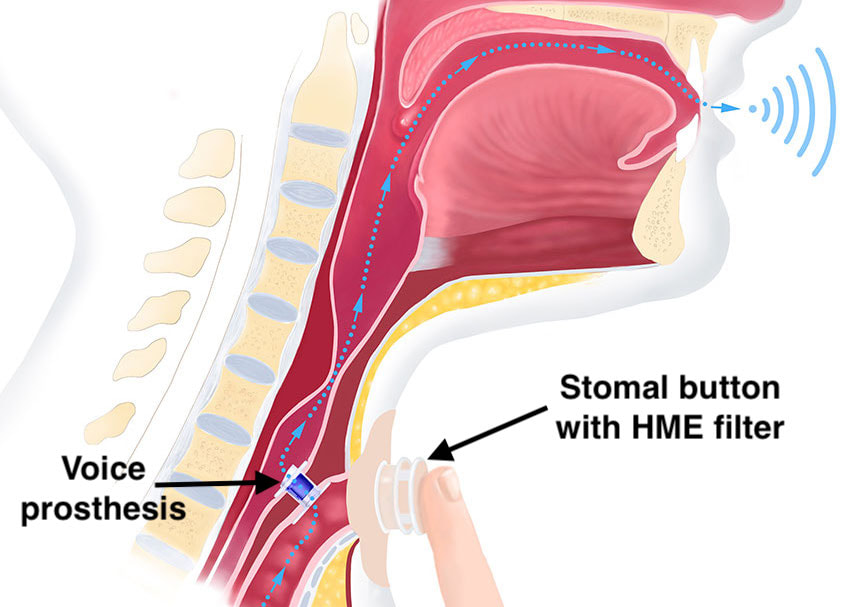
Voice prostheses are short tubes with flanges placed between the trachea and esophagus to allow speech in laryngectomy patients. The devices typically contain a one-way flap valve that prevents esophageal contents from entering the trachea. The patient speaks by exhaling while temporarily occluding the tracheal stoma, thus opening the prosthetic valve and allowing air to enter the pharynx and mouth for speech. Most voice prostheses are made entirely of plastic and are MR Safe. Others, like the Blom-Singer® Advantage (Inhealth Technologies), contain a titanium valve and are MR Conditional. The Provox® Activalve (Atos Medical) is unique in that it contains small magnets and is rated MR Unsafe by the manufacturer. The actual measured displacement forces at 1.5T are actually minimal, however, and would not likely cause dislodgment.
|
Instead of occluding the stoma with one's finger, a speech valve may be fitted over the stoma that spontaneously closes in response to forcible exhalation during attempted speech. Some trachostoma valves, like the Blom-Singer ATSV II (Inhealth Technologies) are made entirely of silicone. Others, including the Provox® FreeHands FlexiVoice™ (Atos Medical) and the ESKA Window® II (Servona) contain small magnets and are considered MR Unsafe.
|
Laryngeal stents are used for short-term stabilization after laryngeal surgery or trauma and to prevent stenosis. To my knowledge all current models are made entirely of silicone and hence MR Safe.
|
Laryngeal implants are used to bring paralyzed vocal cords into a more median position. Nearly all such implants are either injectable biomaterials or surgically implanted non-metals (hydroxyapatite, silicone) and are thus MR Safe. The only exception is the Titanium Vocal Fold Medialization Implant (TVFMI, Kurz Medical) used more commonly in Europe than the USA. The TVFMI is MR Conditional up to 7.0T.
|
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Grady RE Jr, Perkins WJ. An unexpected cause of magnetic resonance image distortion: the endotracheal tube pilot balloon. Anesthesiology 1997; 86:993-4.
Hilgers FJM, Ackerstaff AH, Van As CJ, et al. Development and clinical assessment of a heat and moisture exchanger with a multi-magnet automatic tracheostoma valve (Provox FreeHands HME) for vocal and pulmonary rehabilitation after total laryngectomy. Acta Otolaryngol 2003; 123:91-99. [DOI LINK]
Neuberger D, Kress P, Weidig G, et al. Can voice prostheses remain in situ? An inquiry to insure the safety of modern voice prostheses in MRI. Abstract from 81st Annual Meeting of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery. Wiesbaden, May 2010. [DOI LINK]
Simpao AF, Pollock AN, McGinnis SE, Gàlvez JA. The vanishing neck mass: how using a laryngeal mask airway during magnetic resonance imaging of a child can cause misdiagnosis. Paediatr Anaesth 2016; 26:942-3. [DOI LINK]
Sittel C. Larynx: implants and stents. GMS Curr Top Otorhinolaryngol Head Neck Surg 2009; 8:Doc04. [DOI LINK]
Zaballos M, Bastida E, del Castillo T, et al. In vitro study of magnetic resonance imaging artefacts of six supraglottic airway devices. Anesthesia 2010; 65:569-572. [DOI LINK]
Grady RE Jr, Perkins WJ. An unexpected cause of magnetic resonance image distortion: the endotracheal tube pilot balloon. Anesthesiology 1997; 86:993-4.
Hilgers FJM, Ackerstaff AH, Van As CJ, et al. Development and clinical assessment of a heat and moisture exchanger with a multi-magnet automatic tracheostoma valve (Provox FreeHands HME) for vocal and pulmonary rehabilitation after total laryngectomy. Acta Otolaryngol 2003; 123:91-99. [DOI LINK]
Neuberger D, Kress P, Weidig G, et al. Can voice prostheses remain in situ? An inquiry to insure the safety of modern voice prostheses in MRI. Abstract from 81st Annual Meeting of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery. Wiesbaden, May 2010. [DOI LINK]
Simpao AF, Pollock AN, McGinnis SE, Gàlvez JA. The vanishing neck mass: how using a laryngeal mask airway during magnetic resonance imaging of a child can cause misdiagnosis. Paediatr Anaesth 2016; 26:942-3. [DOI LINK]
Sittel C. Larynx: implants and stents. GMS Curr Top Otorhinolaryngol Head Neck Surg 2009; 8:Doc04. [DOI LINK]
Zaballos M, Bastida E, del Castillo T, et al. In vitro study of magnetic resonance imaging artefacts of six supraglottic airway devices. Anesthesia 2010; 65:569-572. [DOI LINK]
Related Questions
What do you do about tracheobronchial airway devices like stents, valves and coils?
What do you do about tracheobronchial airway devices like stents, valves and coils?