In addition to the obviously brighter signal from fat seen in FSE/TSE imaging, several other subtle differences in contrast may be noted compared with conventional SE imaging. FSE differences include: 1) decreased sensitivity to susceptibility; 2) increased magnetization transfer effects; and 3) edge and contrast effects.
|
Decreased Sensitivity to Susceptibility
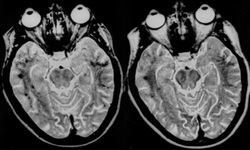
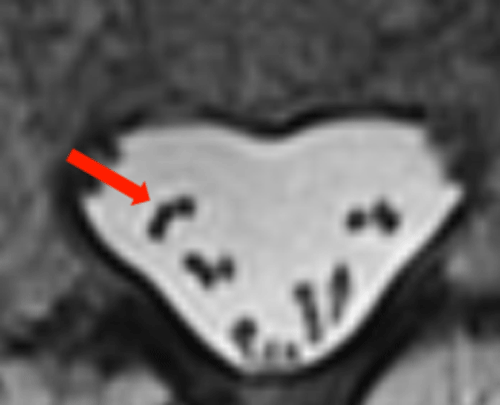
An illustrative example of this phenomenon is seen on routine T2-weighted brain images (right). At field strengths of 1.0T and higher, low signal is commonly noted in the basal ganglia, substantia nigra, and red nuclei secondary to accumulation of intracellular iron in these structures. Microscopic iron deposits cause local distortions of the magnetic field, known as magnetic susceptibility gradients. When water molecules diffuse through these gradients, accelerated spin dephasing and T2*- shortening occurs, reducing the MR signal.
The degree of signal loss depends on the time diffusing water spends in the inhomogeneous fields before refocusing by 180°-pulses. In CSE, the time for refocusing is determined by echo time (TE), which may be 50 ms or longer. In FSE, however, 180°-refocusing pulses occur at very short intervals, determined by ESP (typically 5-10 ms), allowing much less time for susceptibility-induced dephasing. |
|
Diminished sensitivity to susceptibility makes FSE the sequence of choice whenever surgical hardware or metallic foreign bodies are present, significantly reducing artifacts. This advantage may turn into a disadvantage, however, whenever detection of susceptibility changes within tissue are needed for diagnosis, such as for detection of calcification or hemorrhage.
|
Increased Magnetization Transfer Effects
As described in a prior Q&A, magnetization transfer is a process by which spins in a pool of "free" water exchange energy with those in a pool of macromolecules and "bound" water. The multiple 180°-refocusing pulses in FSE provide off-resonance saturation to the macromolecular pool, causing it to become progressively saturated. In brain tissue, the macromolecular pool consists of myelin and membrane phospholipids with very short T2's that are not directly imaged. With increasing number of pulses, the magnetization transfer effect becomes more pronounced, with white matter especially becoming noticeably darker. There is a minor signal loss from gray matter as well. Conversely, the signals from cerebrospinal fluid and fat are not suppressed and these substances appear somewhat brighter than brain.
Edge and Contrast Effects (k-space Filtering)
As illustrated in the examples above, FSE and CSE are similar but structurally different pulse sequences. Even with TR fixed and TE=TEeff, the relative signal intensities of various tissues will not be the same. We have already described several physical phenomena (disruption of J-coupling, decreased sensitivity to susceptibility, and magnetization transfer) that explain some of the differences.
|
An additional important phenomenon (applicable to FSE and all rapid/echo-planar techniques) is called k-space filtering. The fundamental idea is that T2 decay is occurring during each TR interval while the lines of k-space are being filled. Each echo is thus collected at a different point along the T2-decay curve. The lines of k-space are thus weighted unevenly and image contrast is constantly changing throughout the acquisition.
|
Overall image contrast is determined by data acquired near the center of k-space (at TEeff), while early and later echoes reflect high spatial frequency information from the periphery of k-space. These peripheral lines, obtained at different TE values, have signal intensities modulated by T2 decay, resulting in blurring of the image. The more echoes collected (i.e., the longer the ETL), the greater will be the blurring.
The most spatial blurring occurs when TEeff is short, because the higher order phase encode steps that provide edge detail are being filled with echoes late in the train. Thus T1- and proton-density-weighted images demonstrate the highest degree of blurring. A well-cited clinical example concerns the diagnosis of subtle knee meniscal tears which may be blurred out on proton-density-weighted FSE images.
Fluids (like CSF) have long T2 relaxation times, and so there is relatively little change in signal intensity between early and late echoes. Hence for T2-weighted FSE imaging of long T2 materials k-space blurring is not much of a problem. For most solid tissues, however, T2 is shorter there is a significant difference in signal intensity between early and late echoes. Hence more spatial blurring is expected on T2-weighted FSE images of solid, short-T2 tissues. Such blurring may potentially result in failure to detect small objects or lesions with T2 values close to background.
When long and short T2 tissues are juxtaposed, an interesting paradoxical phenomenon called pseudo-edge enhancement may occur. Pseudo-edge enhancement is most commonly seen on axial T2-weighted FSE images of the lumbar spine as white "halos" surrounding nerve roots.
The mechanism is illustrated below and results from blurring (alteration of the image point-spread function) for the nerve root and CSF components. CSF, with long intrinsic T2, does not show much blurring, but nerve roots (with short T2's) do. The blurred signals from the nerve roots spill over into the adjacent high signal CSF, increasing brightness in the vicinity. Even when a halo is not seen, pseudo-edge enhancement explains why nerve roots in the thecal sac are so sharply delineated of FSE images.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Garyun BB, Major NM, Helms CA. Comparison of fast spin-echo versus conventional spin-echo MRI for evaluating meniscal tears. Am J Roentgenol 2005; 184:1740-1743.
Jones KM, Mulkern RV, Mantello MT, et al. Brain hemorrhage: evaluation with fast spin-echo and conventional dual spin-echo images. Radiology 1992;182:53-58.
Reimer P, Allkemper T, Schuierer G, Peters PE. Brain imaging: reduced sensitivity of RARE-derived techniques to susceptibility effects. J Comput Assist Tomogr 1996;20:201-205.
Tartaglino LM, Flanders AE, Vinitski S, et al: Metallic artifacts on MR images of the postoperative spine: Reduction with fast spin-echo techniques. Radiology 1994;190:565-569.
Thomas DL, De Vita E, Roberts S, et al. High-resolution fast spin-echo imaging of he human brain at 4.7T: implementation and sequence characteristics. Magn Reson Med 2004;51:1254-1264.
Garyun BB, Major NM, Helms CA. Comparison of fast spin-echo versus conventional spin-echo MRI for evaluating meniscal tears. Am J Roentgenol 2005; 184:1740-1743.
Jones KM, Mulkern RV, Mantello MT, et al. Brain hemorrhage: evaluation with fast spin-echo and conventional dual spin-echo images. Radiology 1992;182:53-58.
Reimer P, Allkemper T, Schuierer G, Peters PE. Brain imaging: reduced sensitivity of RARE-derived techniques to susceptibility effects. J Comput Assist Tomogr 1996;20:201-205.
Tartaglino LM, Flanders AE, Vinitski S, et al: Metallic artifacts on MR images of the postoperative spine: Reduction with fast spin-echo techniques. Radiology 1994;190:565-569.
Thomas DL, De Vita E, Roberts S, et al. High-resolution fast spin-echo imaging of he human brain at 4.7T: implementation and sequence characteristics. Magn Reson Med 2004;51:1254-1264.
Related Questions
What is magnetization transfer?
What is magnetization transfer?