|
Invisible In-Canal Hearing Aids
A number of companies produce miniaturized hearing aids that fit deeply within the external auditory canal and are "invisible" except by otoscopic examination. One of the most popular is the extended-wear Lyric (Phonak) device that requires insertion by an audiologist or other hearing professional. Because they contain metal and electronic components, invisible hearing aids (like their non-invisible cousins) must be removed prior to MRI.
|
Typanostomy Tubes
Tympanostomy (ventilation) tubes are devices placed through a surgically created hole in the eardrum to allow drainage of middle ear effusions and to equalize pressures between the middle and outer ear. They are commonly referred to as PE Tubes (where PE = "pressure-" or "pneumatic-equalizing").
The overwhelming majority are made of fluoroplastic, polyethylene, or silicone and are therefore MR Safe. A small number of metallic tubes made of titanium, gold-platinum, or gilt silver exist; these are considered MR Conditional.
 MR Unsafe tympanostomy tubes
MR Unsafe tympanostomy tubes
Only a tiny fraction of tympanostomy tubes are deemed MR Unsafe, specifically a group of four devices made by Kurz Medical (Dusslingen, Germany). Three of these are "Tuebingen-type" collar-button-style tubes with a ferromagnetic stainless steel wire (the latter component making the device unsafe). The fourth is the so-called "Minimal-type" composed of gold-plated ferromagnetic stainless steel and intended for short-term use.
Ossicular Implants
The middle ear contains three bony ossicles (malleus, incus, and stapes) that mechanically transmit vibrations of the eardrum to the inner ear where sensorineural hearing occurs. A number of implants are available to replace individual ossicles (such as those affected by congenital anomaly, infection, or trauma). Some prostheses supplant two or more ossicles simultaneously: the PORP (Partial Ossicular Replacement Prosthesis) replacing the malleus and incus (but not the stapes); and the TORP (Total Ossicular Replacement Prosthesis) that replacing all three.
|
Ossicular implants are made of various materials, including "MR Safe" non-metals (fluoroplastic and hydroxyapatite) and MR Conditional metals (titanium, Nitinol, nonferromagnetic stainless steel, and platinum). In the history of MRI there has only been a single MR Unsafe ossicular implant ever produced — the McGee stapes prosthesis — accidentally made of ferromagnetic stainless steel.
|
Fascinating details of the story are recounted by Fritsch in the References. Briefly, in 1987 the manufacturer mistakenly produced 28 lots of McGee prostheses out of ferromagnetic (instead of the usual nonferromagnetic) stainless steel ingots. A worldwide recall was initiated and 64 patients were identified who had been implanted; all were issued warning cards not to undergo MR imaging. Of the several patients scanned before receiving the warning, only one documented case of hearing loss secondary to magnetically induced movement of the implant occurred. No other incidents have been reported in the last 30 years.
|
Endolymphatic Shunts
Placement of a small shunt catheter in the endolymphatic duct of the mastoid bone via a microsurgical approach is one of several surgical treatments for severe Meniere's disease. Existing shunts composed entirely of silastic are "MR Safe" while a few models containing a stiff platinum drainage tube are considered MR Conditional.
|
Active Middle Ear Implants
Electromagnetic force transducers placed in the middle ear have been developed to augment sound wave pressure and frequency response of the conductive chain. Worldwide there are four currently marketed devices in this category, two MR Unsafe and two MR Conditional.
|
The MAXUM™ System (Ototronix) consists of a tiny rare-earth permanent magnet attached to the ossicular chain and a removable sound processor in the external auditory canal. The processor uses electromagnetic pulses to vibrate the implant which directly stimulates the inner ear and cochlea. Because of high torque and displacement force on the permanent magnet component, the MAXUM System is considered MR Unsafe.
|
 "MR Conditional" Vibrant Soundbridge™ System
"MR Conditional" Vibrant Soundbridge™ System
The Vibrant Soundbridge™ System (Med-EL) consists of 1) an external audio processor containing a magnetic microphone; 2) an adjacent subcutaneous receiver coil and circuitry transmitting pulses along a wire to 3) a vibrating magnetic transducer in the middle ear. The transducer can be attached to an ossicle/PORP/TORP or placed in the round window niche. Both the original version of the Soundbridge and its successor (Model 502) are considered MR Unsafe. (Clinical MR testing of the 502 model, initially thought to be safe from in vitro studies, resulted in discomfort and dislocation of the transducer in several patients). Following redesign the new version (Model 503) is now deemed MR Conditional at 1.5T with no adverse effects noted in clinical trials to date. Note that the magnets in both the audio processor and transducer create substantial artifacts, limiting evaluation of the temporal bone and adjacent brain.
|
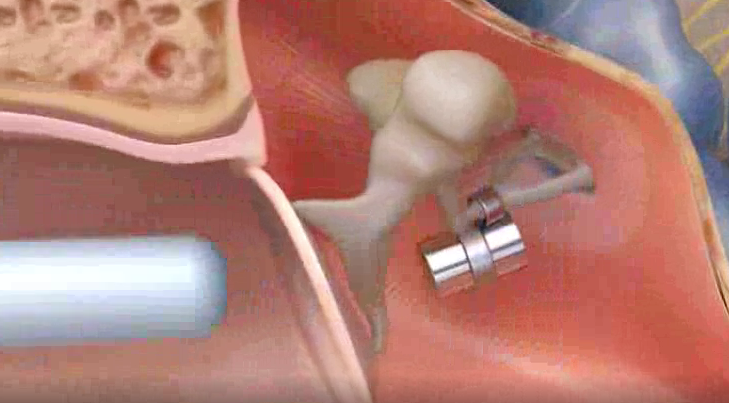
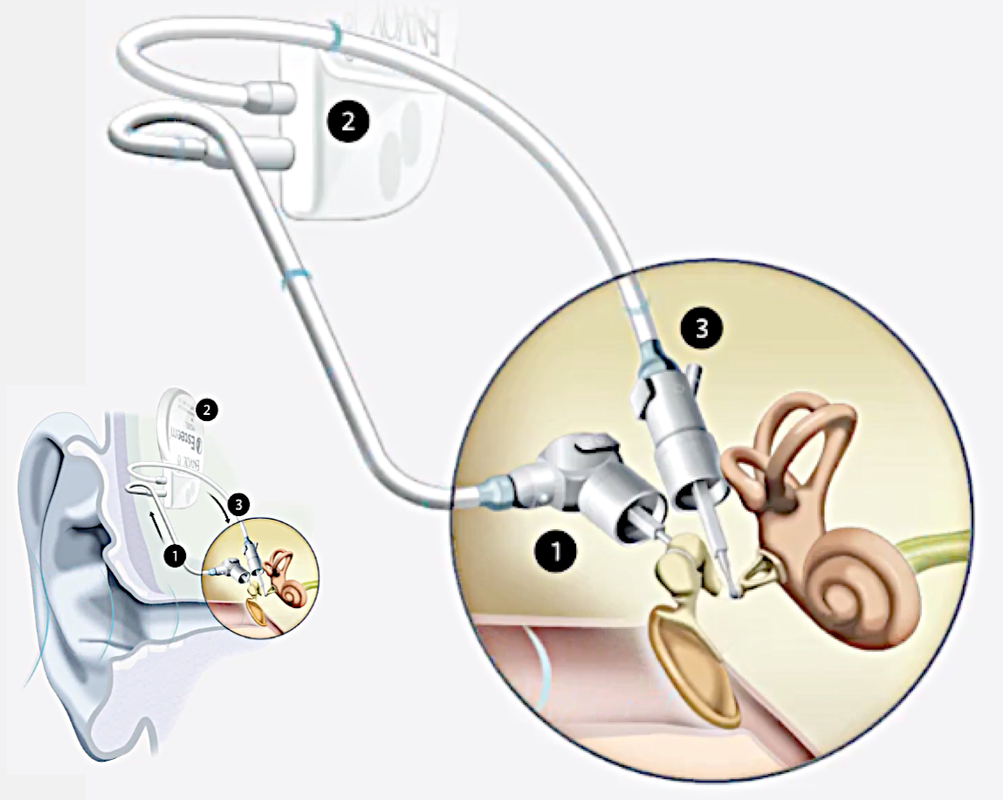
The Envoy Esteem (Envoy Medical) is an MR Conditional fully implantable internal hearing aid with no external components. As shown in the diagram, the Esteem sensor (1) receives sound vibrations from incus, transmitting these to a subcutaneously implanted programmable sound processor (2) that converts the sound into optimized impulses. These intensified signals are transmitted to the driver (3) which vibrates to excite the cochlea. The sensor and driver utilize piezoelectric technology, so no implanted magnets are present. The Esteem sensor does contain a lithium battery, however.
|
|
The Carina® System (Cochlear, Ltd), like the Esteem, is fully implantable device. It is not FDA approved for use in the US, but is available in Europe, Asia, Africa, and Australia. The Carina® receives sensory input from a subcutaneous microphone which relays signals to a separate sound processor with inductive battery recharger which are jointly embedded in the temporoparietal bony bed. Processed signals are then relayed to a middle ear electromechanical actuator/transducer that sends vibrations into the cochlea. Because of these multiple magnetic components, the Carina® System is MR Unsafe.
|
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Azadarmaki R, Tubbs R, Chen DA, Shellock FG. MRI information for commonly used otologic implants: review and update. Otolaryngol Head Neck Surg 2014;150:512-9. [DOI LINK]
Beutner D, Hüttenbrink K-B. Passive and active middle ear implants. GMS Curr Top Otorhinolaryng Head Neck Surg 2009; 8:1-19.
Channer GA, Eshraghi AA, Xue-zhong LIU. Middle ear implants: historical and futuristic perspective. J Otol 2011; 6:10-18. (good review of a number of legacy active middle ear devices from the 1990s to early 2000s such as the Soundtech and TICA that are no longer in production but potentially could be encountered in practice)
Fritsch MH. MRI scanners and the stapes prosthesis. Otol Neuro 2007; 18:733-738. [DOI Link] (includes an interesting more complete story about the McGee prosthesis production and recall)
Seidman MD, Janz TA, Shohet JA. Totally implantable active middle ear implants. Otolarngol Clin N Am 2019; 52:297-309. [DOI LINK]
Todt I, Mittmann P, Ernst A, et al. In vivo experiences with magnetic resonance imaging scans in Vibrant Soundbridge type 503 implantees. J Laryngol Otol 2018; 132:401-403. [DOI LINK]
Azadarmaki R, Tubbs R, Chen DA, Shellock FG. MRI information for commonly used otologic implants: review and update. Otolaryngol Head Neck Surg 2014;150:512-9. [DOI LINK]
Beutner D, Hüttenbrink K-B. Passive and active middle ear implants. GMS Curr Top Otorhinolaryng Head Neck Surg 2009; 8:1-19.
Channer GA, Eshraghi AA, Xue-zhong LIU. Middle ear implants: historical and futuristic perspective. J Otol 2011; 6:10-18. (good review of a number of legacy active middle ear devices from the 1990s to early 2000s such as the Soundtech and TICA that are no longer in production but potentially could be encountered in practice)
Fritsch MH. MRI scanners and the stapes prosthesis. Otol Neuro 2007; 18:733-738. [DOI Link] (includes an interesting more complete story about the McGee prosthesis production and recall)
Seidman MD, Janz TA, Shohet JA. Totally implantable active middle ear implants. Otolarngol Clin N Am 2019; 52:297-309. [DOI LINK]
Todt I, Mittmann P, Ernst A, et al. In vivo experiences with magnetic resonance imaging scans in Vibrant Soundbridge type 503 implantees. J Laryngol Otol 2018; 132:401-403. [DOI LINK]
Related Questions
Can you scan someone with a cochlear implant?
Can you scan someone with a cochlear implant?