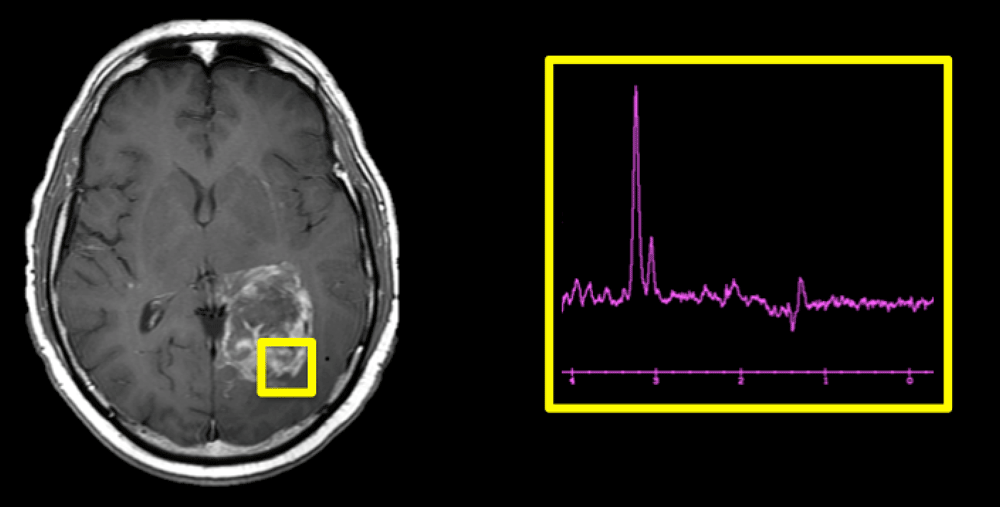
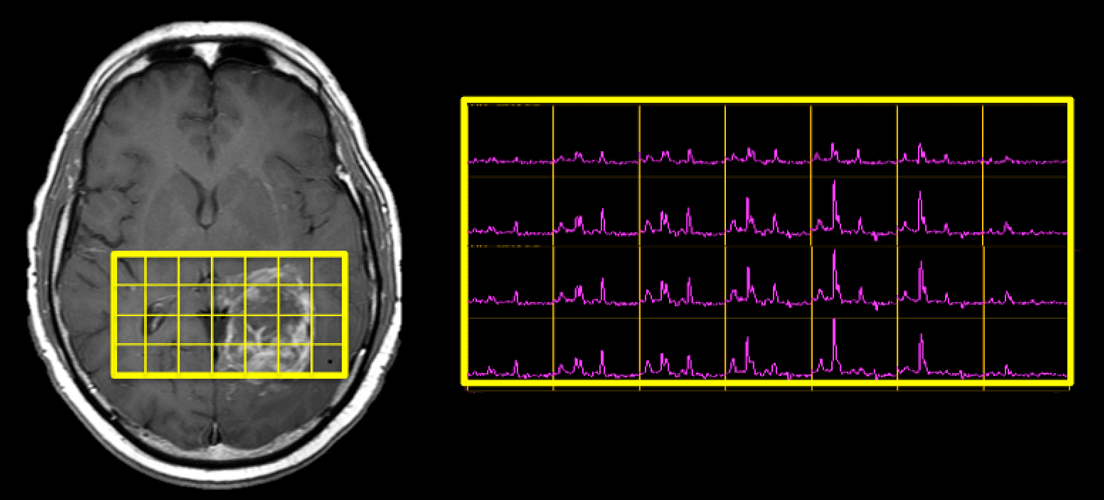
When prescribing an MR spectroscopy exam, one of the fundamental decisions is whether to acquire data from a single voxel or from multiple voxels.
Single-voxel spectroscopy (SVS) techniques are the simplest to acquire and interpret, and hence are the most widely used. They provide high signal-to-noise in a relatively short scan time. Because the imaged region is compact, excellent shimming can be obtained with resultant high-quality spectra suitable for quantitative analysis.
Multi-voxel Chemical Shift Imaging (CSI) techniques offer two potential advantages over SVS: 1) a larger total coverage area (since the size of the entire multivoxel slab is greater), and 2) higher spatial resolution (since the individual voxels are smaller). A wide coverage area is important for large, heterogenous lesions like the brain tumor shown above, where the SVS technique provides data from only a small portion of the mass.
The smaller size of individual voxels in a multi-voxel CSI study may be critical when evaluating small organs like the prostate, where SVS is impractical. Smaller voxels are also advantageous when studying small or irregularly shaped anatomic structures even in larger organs like the brain. Here partial volume effects are reduced by excluding unwanted structures (such as other nuclei, CSF or scalp fat) from the spectral region.
Disadvantages of multi-voxel CSI include: 1) Longer set-up and imaging time; 2) difficulties obtaining homogenous shim over the entire region; 3) lower signal-to-noise and spectral quality for individual voxels; 4) spectral contamination from adjacent voxels.
Advanced Discussion (show/hide)»
No supplementary material yet. Check back soon!
References
Bertoldo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy. Introduction and overview. Neuroimag Clin N Am 2013; 23:359-380.
Moonen CT, von Kienlin M, van Zijl PC, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed 1989; 2:201–207.
Öz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014; 270:658-679.
Posse S, Otazo R, Dager SR, Alger J. MR spectroscopic imaging: principles and recent advances. J Magn Reson Imaging 2013; 37:1301-1325.
Skotch A, Jiru F, Bunke J. Spectroscopic imaging: basic principles. Eur J Radiol 2008; 67:230-239.
Bertoldo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy. Introduction and overview. Neuroimag Clin N Am 2013; 23:359-380.
Moonen CT, von Kienlin M, van Zijl PC, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed 1989; 2:201–207.
Öz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014; 270:658-679.
Posse S, Otazo R, Dager SR, Alger J. MR spectroscopic imaging: principles and recent advances. J Magn Reson Imaging 2013; 37:1301-1325.
Skotch A, Jiru F, Bunke J. Spectroscopic imaging: basic principles. Eur J Radiol 2008; 67:230-239.
Related Questions
If frequency-encoding cannot be used to determine spatial position, how do you localize an MRS signal?
If frequency-encoding cannot be used to determine spatial position, how do you localize an MRS signal?