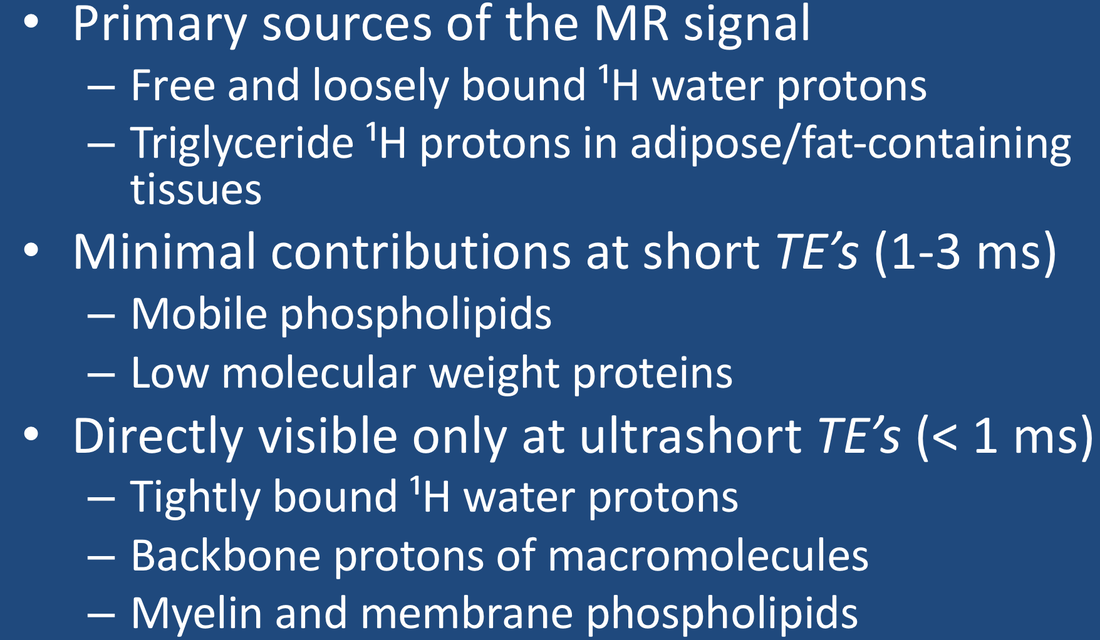
At this point it is worth summarizing the principal sources of the signal in ¹H MR imaging and spectroscopy. The relative contributions of each source depend on the tissue studied and the particular pulse sequence utilized.

The major protons responsible for the MR signal or its modulation: free water, bound water, macromolecules, and fats. Lipid stores are usually in isolated compartments hence the dotted line. Small organic molecules make a very minor contribution to overall signal (except in water suppressed MRS) and are not pictured.
- Water. ¹H2O protons are the largest and most important source of the NMR signal in biological tissues. In a human cell, there are about as many water protons as there are all ¹H protons in lipids and macromolecules combined. As described in the prior Q&A, most cellular water is unstructured and relatively free, but some is either loosely or tightly bound to proteins and other macromolecules. The most tightly bound of water protons have highly restricted motions with very short T2 values (< 1 ms), so that their signals may not be detected with conventional MRI techniques using TE values > 1 ms. Special techniques (ultrashort TE imaging) are required to record the signal from these protons.
- Lipids. Triglycerides, cholesterol esters, and free fatty acids are nonpolar and largely immiscible with water, being stored in specialized fat cells (adipocytes) or as vacuoles/inclusions in other cells. Such fats exist in a semi-liquid state, with slightly restricted motions of their long side chains resulting in short T1 values (a few hundred ms) and moderate to short T2 values (a few dozen ms). Fats are also abundant in cell membrane bi-layers, over half of which is phosphatidylcholine, which has more restricted motions. Lipid-containing cell membranes and myelin have highly restricted motions and T2 values shorter than 1 ms, with signals decaying too quickly to be detected on routine MR imaging.
- Small organic molecules. MR signals are emitted from a wide range of amino acids, sugars, organic acids, low-molecular weight proteins, and the like. These are the primary subject of MR spectroscopy. However, their concentrations are several hundred fold smaller than that of water protons, so their contributions to the overall MR signal is insignificant. In order to observe and measure them in MRS, the water signal must be suppressed.
- Macromolecules. This category includes both large intracellular proteins as well as extracellular connective tissues such as collagen. All macromolecules have ¹H-containing components (e.g., methyl, amide, and methylene groups) from which MR signals may arise. The protons on smaller proteins (say<20 kDa) can more easily rotate and diffuse within cells and possess only moderately short T2 values allowing them to be observable in solution. Larger macromolecules become more immobile and are forced into globular shapes due to cytoplasmic crowding. Their ¹H nuclei have highly restricted motions, being subject to the static and low frequency magnetic fields from neighboring nuclei and paramagnetic ions. Such large macromolecular protons typically have very short T2 values hence may be invisible on conventional MR pulse sequences with TE values longer than 1 ms, thus belonging to the "dark pool" described in the next Q&A.
Advanced Discussion (show/hide)»
The signals from macromolecular protons are not directly imaged on conventional MRI due to their very short T2 values. These nuclei do produce an MR signal, but it decays too quickly to be recorded under normal circumstances. However, several new ultrashort TE (UTE) sequences are now commercially available with more in development allowing the signals from these nuclei to be detected. This exciting research will be described in several later Q&A's.
References
Bydder GM. The Agfa Mayneord lecture: MRI of short and ultrashort T2 and T2* components of tissues, fluids, and materials using clinical systems. Brit J Radiol 2011; 84:1067-1082. (The first published reference I can find where these protons are referred to as "dark matter").
Fullerton GD, Cameron IL, Hunger K, Fullerton HJ. Proton magnetic resonance relaxation behavior of whole muscle with fatty inclusions. Radiology 1985; 155:727-730.
Halle B. Water in biological systems: the NMR picture. In: Bellissent-Funel M-C (ed.). Hydration processes in biology : theoretical and experimental approaches. IOS Press, Clifton, VA. 1999: 233-249.
Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2¹H NMR signals in myelinated nerve: a direct measure of myelin content. Magn Reson Med 2011; 66:24-31.
Span EA, Goodsell DS, Ramchandran R, et al. Protein structure in context: the molecular landscape of angiogenesis. Biochem Mol Biol Education 2013; 41:213-223. (See Figure 7, a great color-coded picture illustrating molecular crowding in cells).
Bydder GM. The Agfa Mayneord lecture: MRI of short and ultrashort T2 and T2* components of tissues, fluids, and materials using clinical systems. Brit J Radiol 2011; 84:1067-1082. (The first published reference I can find where these protons are referred to as "dark matter").
Fullerton GD, Cameron IL, Hunger K, Fullerton HJ. Proton magnetic resonance relaxation behavior of whole muscle with fatty inclusions. Radiology 1985; 155:727-730.
Halle B. Water in biological systems: the NMR picture. In: Bellissent-Funel M-C (ed.). Hydration processes in biology : theoretical and experimental approaches. IOS Press, Clifton, VA. 1999: 233-249.
Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2¹H NMR signals in myelinated nerve: a direct measure of myelin content. Magn Reson Med 2011; 66:24-31.
Span EA, Goodsell DS, Ramchandran R, et al. Protein structure in context: the molecular landscape of angiogenesis. Biochem Mol Biol Education 2013; 41:213-223. (See Figure 7, a great color-coded picture illustrating molecular crowding in cells).
Related Questions
How does the presence of macromolecules affect T1 and T2?
Why are some nuclei "invisible" on MRI?
How does the presence of macromolecules affect T1 and T2?
Why are some nuclei "invisible" on MRI?